In the previous article "Mastering the Art of Stability," we revealed the key factors influencing the physical stability of amorphous solid dispersions (ASDs) and recognized that choosing the appropriate method for preparing ASDs is crucial for successful product development. In this article, we will focus on the classification of ASD preparation methods and how to select the right preparation method for producing ASDs.
Although there are many reported methods for preparing ASDs, the basic principle behind forming amorphous structures is similar. Firstly, the crystal lattice structure of the drug is disrupted by heating or dissolving it in a solvent, converting it into a liquid state. Then, rapid cooling or drying is employed to obtain an amorphous solid dispersion. Broadly speaking, ASD preparation methods are mainly divided into two categories based on different ways of disrupting the crystal lattice: solvent-based methods and melt-based methods. Solvent-based methods include spray drying, electrospraying, fluid bed granulation/drying, supercritical fluid methods, spray freeze drying, solvent casting, etc. Among these, spray drying and fluid bed granulation/drying have been commercialized. Melt-based methods encompass hot melt extrusion, KinetiSol®, microwave heating, melt granulation, ultrasonic compaction, and others. Currently, hot melt extrusion is the most widely commercialized method.
Solvent-Based Methods
ASD preparation through solvent evaporation involves two main steps: the dissolution of the drug and polymer in an organic solvent system, followed by the subsequent evaporation of the solvent to obtain an amorphous solid dispersion of the drug and polymer. Solvent evaporation typically occurs at temperatures below the drug's melting point, making it suitable for thermally sensitive compounds.
When using solvent evaporation methods to prepare ASDs, the selection of the solvent system is crucial. It is necessary to find a solvent system that can dissolve the drug-polymer system and is compatible with it. Additionally, the residual solvent left in the final product must meet regulatory requirements. To achieve the desired solvent properties, a combination of several solvents is often used. Before solvent evaporation, the solvent should not adversely affect the chemical stability of the formulation components. Furthermore, it is essential to minimize the use of Class I solvents and opt for safer Class III solvents. When working with highly toxic organic solvents, it is crucial to thoroughly remove residual solvents from the final product. Consideration should also be given to the impact of toxic solvents on production personnel, necessitating appropriate safety measures and waste disposal procedures.
Melt-Based Methods
If a drug can melt without undergoing degradation at temperatures below 150°C, melt-based methods can be considered. In melt-based methods, a mixture of the drug and polymer is heated to melt and then rapidly cooled to form an ASD. One significant advantage of melt-based methods is the avoidance of using solvents. However, high temperatures during the melting process may lead to drug degradation, making it unsuitable for thermally sensitive drugs. Melt-based methods require the drug to have sufficient solubility and compatibility within the polymer melt. The polymer acts as a medium for dissolving or dispersing the drug and can contribute to stabilizing the amorphous state. Typically, polymers used in these methods should have a low melting point or glass transition temperature (Tg). Commonly used polymers include polyvinylpyrrolidone (PVP), polyvinyl acetate (PVA), polyvinylpyrrolidone/vinyl acetate copolymers (PVP/VA), cellulose esters and ethers, acrylic acid esters, and derivatives of poly(methyl methacrylate). These polymers can be used individually or in combination to improve amorphous stability, solubility, and bioavailability.
During the melting process, plasticizers are sometimes added. Melt-based ASD preparation processes often involve high shear stresses, and the addition of plasticizers can reduce shear stress, decrease the viscosity of the mixture, and lower the processing temperature. Commonly used plasticizers include d-α-tocopherol, Poloxamers (polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymers), low-molecular-weight polyethylene glycols (PEGs), and surfactants. The choice of a plasticizer depends on its intended function, such as reducing processing temperature or lowering melt viscosity. Traditional plasticizer concentrations range from 5% to 30% (by weight), which significantly reduces the drug load. Moreover, the presence of residual plasticizers may impact product performance. Ideal plasticizers should provide the desired plasticizing effect and be easily removable from the formulation, often using low-boiling-point solvents or substances that can be evaporated or sublimated.
Other Methods
In addition to the solvent-based and melt-based methods discussed earlier, wet granulation and co-precipitation are also methods used for preparing ASDs. Both of these techniques have been successfully applied in the commercial production of ASD drugs.
Solvent-Based Methods
For early-stage laboratory research, solvent casting can be used. For larger batch preparations, rotary evaporation or spray drying can be considered. When moving to pilot-scale or commercial production, factors such as process efficiency, yield, particle characteristics, as well as the toxicity, environmental impact, operator safety, and flammability/explosion risks associated with solvents need to be considered. Therefore, the available preparation processes are limited, with spray drying being the primary choice.
Melt-Based Methods
For early-stage laboratory research, melt quenching of ASDs can be performed using containers in differential scanning calorimetry (DSC), or vacuum compression molding (VCM) can be employed. For larger laboratory-scale batches, options include melt quenching using hot plates/oil baths or hot melt extrusion. Materials with viscosities exceeding 300 cP in the melt can pose challenges for ASD processing. In such cases, hot melt extrusion should be given priority. When considering pilot-scale or commercial production, the choice of preparation processes is limited, primarily involving hot melt extrusion.
Figure 1 shows an ASD preparation method decision tree summarized by SV. Bhujbal et al. for reference. It's important to note that the selection of an ASD preparation method should be made by considering a combination of factors, including drug properties, product design and clinical requirements, the development stage and strategy, hardware conditions, and other relevant considerations.
(Figure 1 was mentioned but not provided in the text. You may refer to the original source for the decision tree.)
Figure. 1 Decision tree of ASD preparation method selection
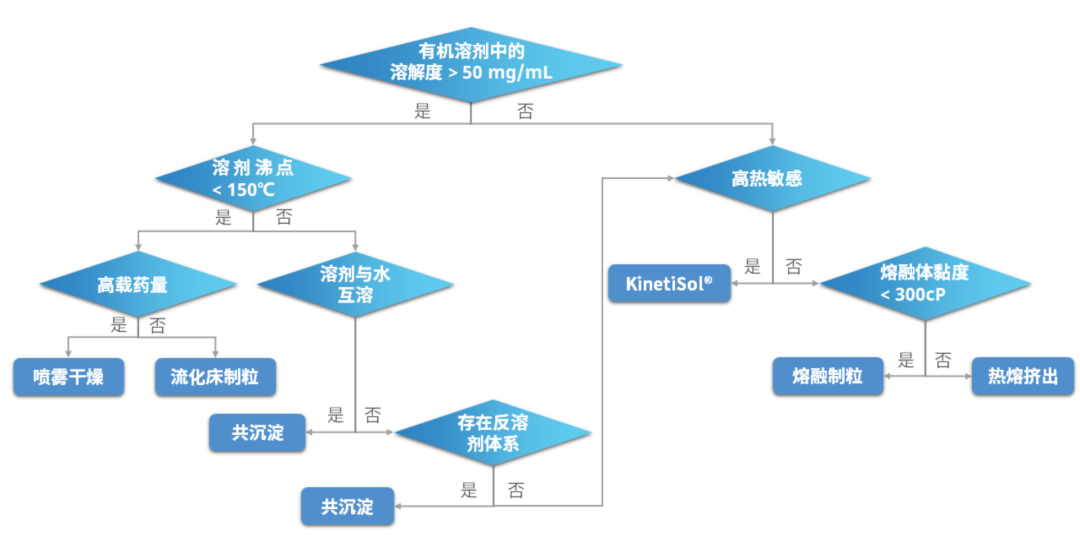
In addition to a drug's thermal stability and solubility, other important factors to consider when choosing a manufacturing method include production batch size and equipment availability.
We have summarized a total of 28 ASD drugs that have been approved by the FDA in the past 20 years. Among these, 11 drugs were prepared using the hot melt extrusion method, 14 drugs were prepared using the spray drying method, and the remaining 3 drugs used other methods. It's evident that hot melt extrusion and spray drying are currently the two most commonly used, technically mature, and commercially implemented ASD production methods.
Crystal Pharmaceutical's CDMO Division (Crystal Pharmaceutical) utilizes advanced international technology in amorphous solid dispersion (ASD), with a focus on both hot melt extrusion and spray drying platforms. We are dedicated to assisting clients in selecting the appropriate ASD preparation methods and processes to address the challenges of poorly soluble drugs and to support your new drug development endeavors.