Developability Assessment
The world needs new medicines and drug developers are racing to bring promising molecules to the market. However, drug development programs stall when:
- Physicochemical properties of API form and formulation are uncertain
- Lead compounds require quantifiable comparison
- Form and formulation guesswork cause incoherent tox exposures
From lead optimization to First in Human (FIH), programs are at risk of hitting significant speed bumps if the physicochemical risks for API form and formulation are never evaluated.
Innovators need a strategic and cohesive approach to move their molecule to medicine.
The Mol2Med™ Developability Assessment is a dynamic, fit-for-purpose service that helps drug developers achieve a comprehensive understanding of their molecule's potential for development.
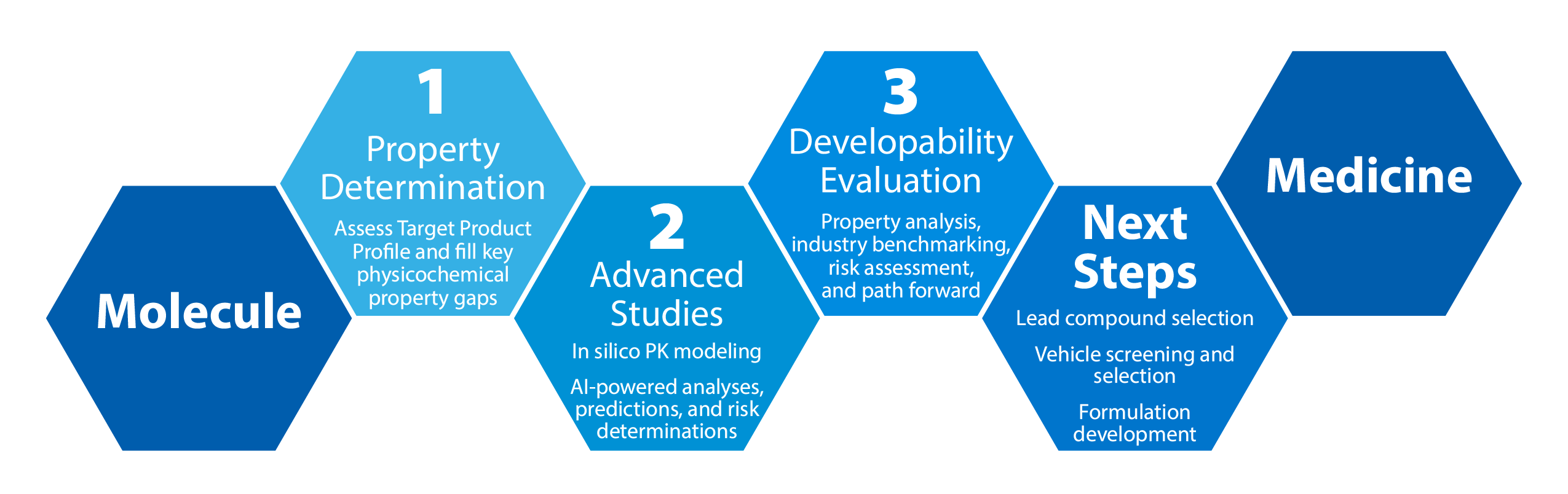
With as little as 500mg of API and 4 weeks, Crystal Pharmatech offers a smarter path forward by:
- Determining the key physicochemical properties of your compound(s)
- Benchmarking according to industry standards
- Providing a high-level approach for achieving most probable success in GLP Tox and FIH
A full understanding of your molecule goes well beyond test results and raw data. Industry benchmarking builds upon decades of efforts and studies to predict in vivo performance. Combined with AI-driven technologies and in silico capabilities, you will gain powerful insights to unlock your molecule's potential and make faster, smarter decisions forward. |
Crystal Pharmatech R&D and Subject Matter Experts have decades of experience moving molecules to medicine.
We derive highly accurate data from novel API-sparing technologies. We run this data through in silico simulations, industry benchmarks, and Crystal Pharmatech experts to give our partners insights that will:
- Expedite your development timeline
- Minimize costs through a strategic scope of work
- Maximize your molecule's likelihood of success in GLP Tox and FIH
Our pathway offers specific next steps to reach all major milestones throughout your drug development program - from lead compound selection to formulation development.
Leading experts will ensure CMC is not limiting Tox and Phase I success while assessing later stage developability risks of API and drug product.
The cohesion between our solid-state and formulation experts ensures continuity and a goal-oriented approach throughout every stage of development.