11 Apr 2025
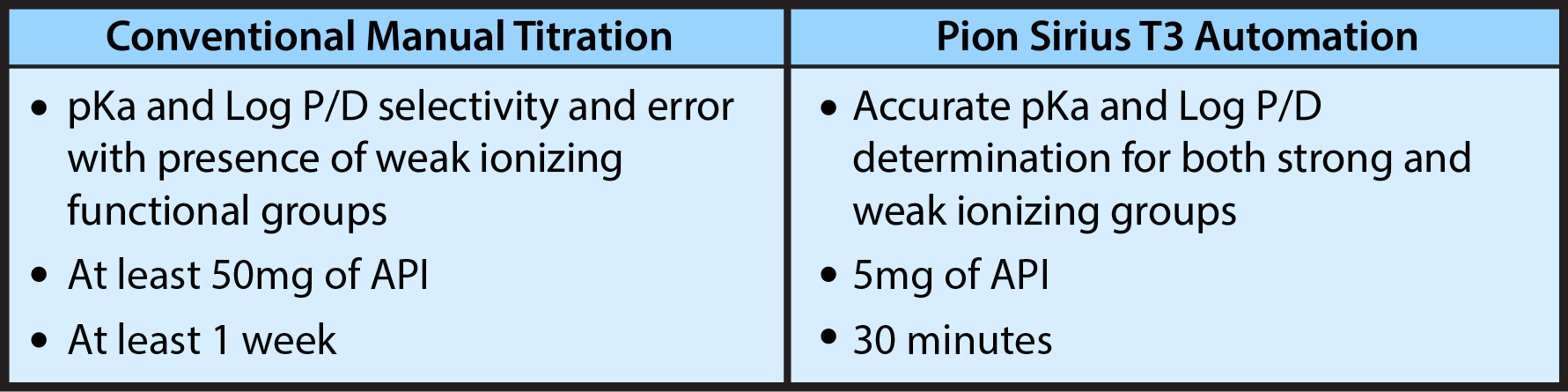
Earlier than ever before in the development timeline, biotech can achieve a comprehensive understanding of their molecule's physicochemical properties. Using material-sparing automation, Crystal Pharmatech guides its partners towards smarter decisions before more time and money is invested in their development process.

A thorough understanding of the drug's pKa and Log P, determined through pH and UV metric titration methods, will:
Guide a fundamental understanding of solid-state interactions and the overall crystalline form.
Allow for more accurate prediction of physical, chemical, and biological in vivo predictions.
Direct better formulation decisions and avoid costly mistakes when given solubility under different pH conditions.