20 Jun 2025
More than 70% of drug candidates worldwide are chiral. Typically, for chiral API, only one enantiomer or diasteromer is biologically active or desirable. Therefore, the production of enantiopure compounds or diastereomers is imperative. In the production of small molecule drugs with desired chirality, separation via crystallization can be much more economical and environmentally favorable than chromatography.
Classical Chiral Resolution
Classical chiral resolution of an enantiomeric acid or base via diastereomeric salt formation and crystallization is a common approach to separate the desired enantiomer from its racemic mixture. From screening for desired resolving agent, polymorph and solvent to design and optimization of crystallization process, we offer solutions catering various specific needs in chiral separation.
Preferential Crystallization
The upper limit of 50% yield to an enantiopure compound by conventional chiral separation methods can be surpassed by combining racemization with crystallization of an enantiomer from its racemic mixture (or conglomerate). Identification of proper racemizing agent and implementation of crystallization engineering effectively aid our successful design and optimization of preferential crystallization processes.
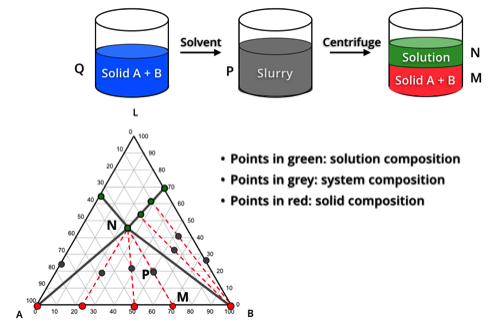
We have established a systematic method for chiral purification or resolution of enantiomers or diastereoisomers by crystallization. Ternary phase diagram is a powerful tool to understand the crystallization and purification process of chiral drugs. Based on ternary phase diagrams of enantiomer or diastereoisomer systems, the feasibility of purifying a chiral drug molecule by crystallization or dissolution can be immediately determined, and the ternary phase diagram can help us find the optimal crystallization process conditions.
.webp)
