Crystal Pharmatech provides a one-stop Clinical Supply Service from pre-clinical to post-marketing.
| One-Stop Clinical Supply Service | |||
|---|---|---|---|
|
|
|
|
| Primary and Secondary Package | |||
|---|---|---|---|
Primary Packaging | GMP environment:
|
|
|
Secondary Packaging |
| Package and Label design:
|
|
| Storage, distribution, return and destruction | |||
|---|---|---|---|
| Storage | Distribution | Return | Destruction |
|
|
|
|
| Covering China Mainland, China Taiwan, Australia, New Zealand, Europe, the United States and Canada | |||
International transportation
Importation & Exportation
Import permit/CIQ application
Custom clearance
Cold chain Global network
Value Added Service
Comparator Sourcing
Ancillary Sourcing
Clinical Supply Optimization
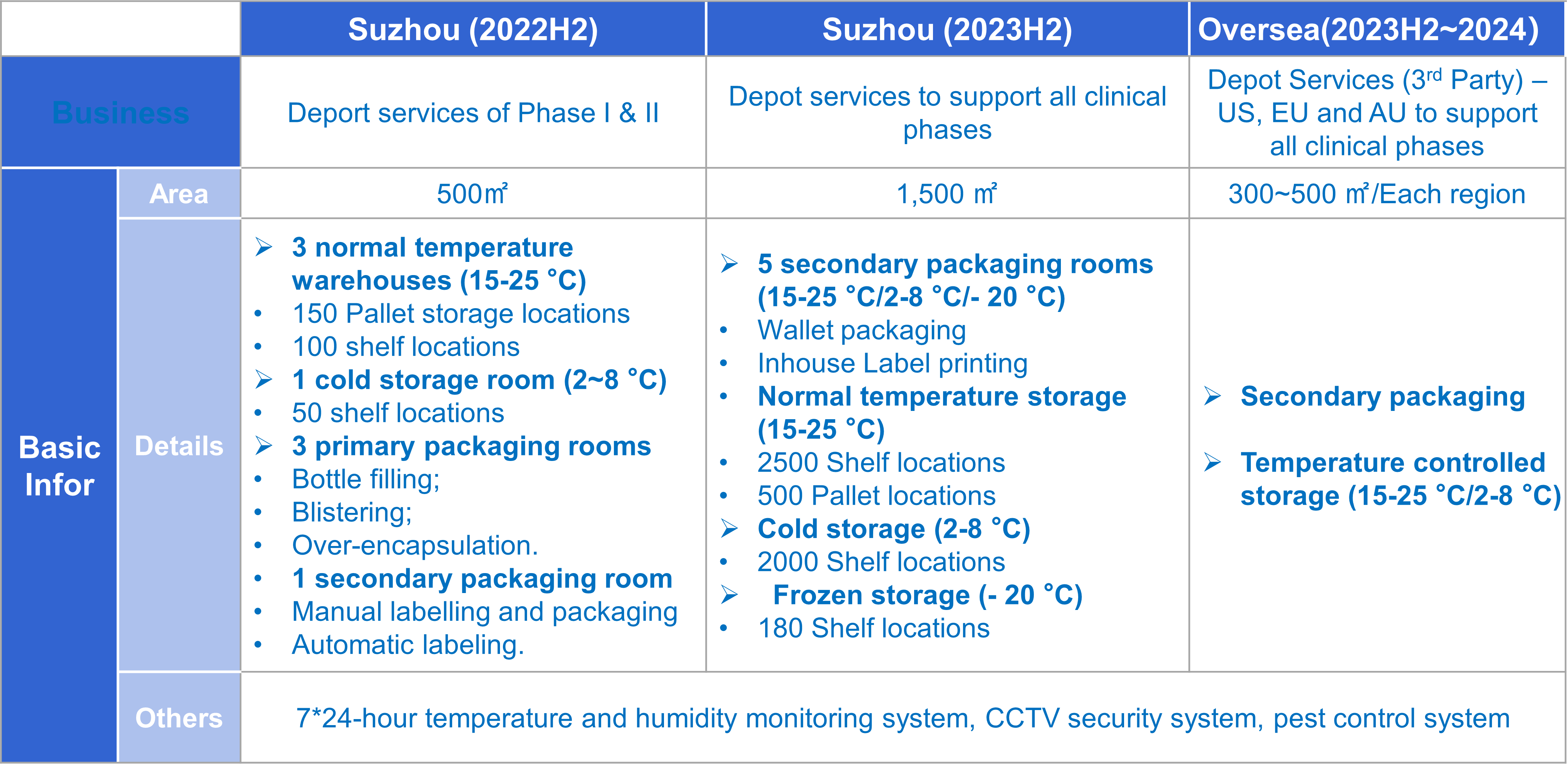
Capacity
Have a question or need support with your project? Please complete the form, and our team will get back to you shortly.
Our capabilities span three specialized platforms:
Small Molecule
Crystal Bio Solutions
Crystal NAX
By providing your e-mail address, you agree to receive an e-mail response from Crystal Pharmatech to your inquiry. The information you submit will be governed by our Privacy Policy.
Subscribe to be the first to get the updates!