Amorphous solid dispersion (ASD) offers enhanced solubility over crystalline forms due to its elevated Gibbs free energy. Additionally, ASD is capable of maintaining high levels of supersaturation in vivo, positioning it as one of the most effective approaches for improving the dissolution rate and bioavailability of poorly soluble drugs. In the previous article, we explored common techniques for characterizing ASD, such as X-Ray Powder Diffraction (XRPD), Differential Scanning Calorimetry (DSC), Polarized Light Microscopy (PLM), Scanning Electron Microscopy (SEM), Infrared Spectroscopy (IR), Raman Spectroscopy (RM), and Solid-state Nuclear Magnetic Resonance (ssNMR). After characterization, it is essential to evaluate ASD’s solubility, dissolution rate, and stability to determine its suitability for further development. This section will focus on the comprehensive evaluation of ASD.
The dissolution behavior of solid dispersions is influenced by a complex interplay of factors including the properties of the drug, the drug-loading rate, the type of carrier used, the drug-carrier-surfactant interactions, as well as dissolution conditions. Polymers, frequently employed as carriers in pharmaceutical Amorphous Solid Dispersions (ASD), play a crucial role in maintaining the supersaturation state of the dissolution system. They achieve this through mechanisms such as fostering drug-polymer interactions and enhancing the solution's viscosity. These actions help to prevent the surface crystallization of amorphous drugs, thereby significantly improving the dissolution behavior of ASDs. Furthermore, the choice of different carriers can influence the rate and site of drug release, allowing for tailored release profiles that meet specific therapeutic needs.
Figure 1 presents a comparative analysis of the dissolution profiles of crystalline Felodipine and Felodipine Amorphous Solid Dispersions (ASDs) prepared by spray drying with various carriers in a pH 6.8 buffer solution. The crystalline form of Felodipine shows relatively low dissolution under this condition. In contrast, ASD formulations using HPMCAS as the carrier not only achieve higher solubility but also sustain longer-lasting supersaturation. However, formulations with PVP K30 as the carrier demonstrate high dissolution within the first 15 minutes, which is quickly followed by a marked decrease. X-Ray Powder Diffraction (XRPD) analysis conducted on the solid samples during dissolution indicates that ASDs with PVP K30 as the carrier tend to recrystallize rapidly during the dissolution process, contributing to the observed reduction in solubility. This analysis underscores the importance of carrier selection in optimizing the dissolution behavior and stability of ASDs.
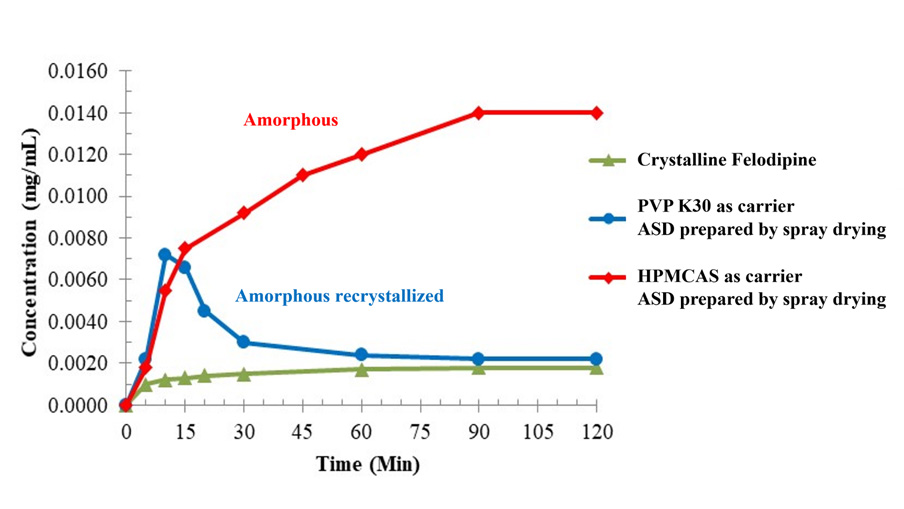
Figure 1: Comparison of the Dissolution of Crystalline Felodipine and Felodipine ASDs Prepared with Different Carriers in pH 6.8 Buffer Solution
Amorphous Solid Dispersion (ASD) is susceptible to recrystallization and phase separation, influenced by both intrinsic factors—like molecular mobility, crystallization kinetics, and drug-carrier interactions—and external environmental factors, including humidity, temperature, surface conditions, and varying preparation methods. Given these susceptibilities, it is critical to rigorously assess the stability of ASDs. While the assessment of chemical stability for ASDs follows protocols similar to those used for conventional formulations and will not be covered here, this section will focus specifically on the physical stability of ASDs. This involves evaluating how well ASDs maintain their amorphous structure under different storage and handling conditions, which is essential for ensuring their efficacy and shelf life.
To mitigate self-crystallization stemming from intrinsic factors, it is crucial to manage the drug-loading rate to prevent reaching a saturation point within the carrier. Moreover, different carriers possess unique properties in terms of plasticization and anti-crystallization effects, making stability assessments essential for comparing and selecting the optimal formulation. Table 1 summarizes the stability data of Felodipine Amorphous Solid Dispersions (ASDs) formulated with varying drug-loading rates (20% and 40%) and stored for three months under both long-term and accelerated conditions. The characterization data reveals that ASDs with a 20% drug loading rate maintain robust physicochemical stability under the evaluated conditions. Conversely, ASDs with a 40% drug loading rate exhibit signs of Felodipine recrystallization after three months, as evidenced by X-Ray Powder Diffraction (XRPD) and Polarized Light Microscopy (PLM) analyses. This indicates that ASDs with higher drug loading rates tend to have comparatively poorer physical stability, which could adversely impact their dissolution and bioavailability. Consequently, ASD formulations with a 20% drug loading rate are identified as more favorable due to their enhanced stability profiles.
External environmental factors, such as temperature and humidity, significantly influence the physical stability of Amorphous Solid Dispersions (ASDs). Given that many ASD carriers are hydrophilic materials with hygroscopic properties, environmental moisture can act as a plasticizer, thereby lowering the system's glass transition temperature (Tg) and altering its physical properties, which may reduce drug efficacy. Moisture can also compete with the drug for binding sites on the carrier materials, potentially hindering effective drug-carrier interactions and facilitating phase separation. Moreover, elevated temperatures have been shown to increase molecular mobility within the system, thereby accelerating the crystallization process. Consequently, after selecting an appropriate carrier, it is essential to rigorously control environmental temperature and humidity to prevent drug recrystallization and ensure sustained physical stability of the ASD. Such measures are critical to maintaining the integrity and therapeutic effectiveness of the formulation under various storage conditions.
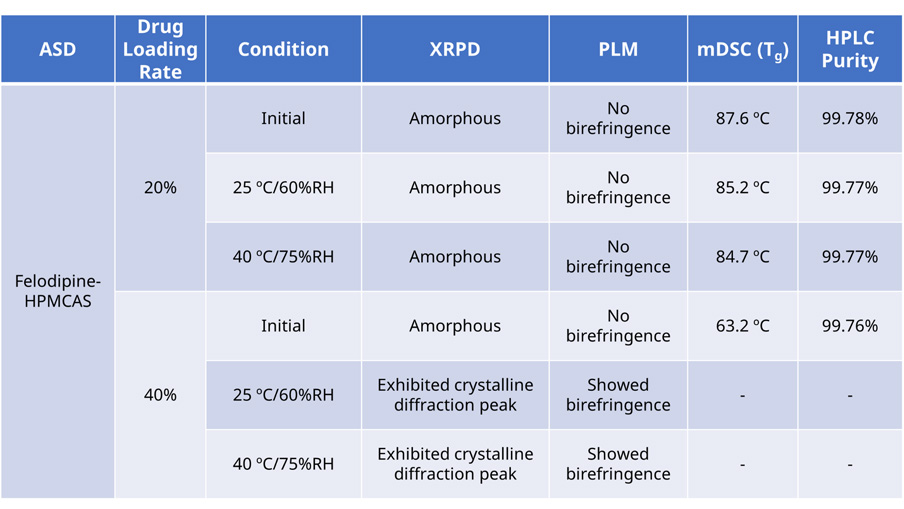
Table 1:Stability Assessment of Felodipine ASDs prepared with different drug-loadings rate (20% and 40%) after storage for three months under long-term and accelerated conditions.
The physicochemical stability of Amorphous Solid Dispersion (ASD) products under various storage conditions, including accelerated and long-term scenarios, is instrumental in determining the most appropriate storage parameters. Monitoring and analyzing the physicochemical properties during stability assessments is crucial and can effectively be achieved using the earlier mentioned characterization and evaluation techniques. This comprehensive analysis helps ensure that the ASD maintains its intended efficacy and safety profiles throughout its shelf life.
In recent years, the development of poorly soluble compounds has surged, with Amorphous Solid Dispersion (ASD) gaining significant attention for its enhanced solubility and bioavailability. However, ASDs often have more complex compositions than crystalline drugs, necessitating accurate and rapid characterization and evaluation. It is important to note that utilizing a combination of experimental techniques can yield a more comprehensive understanding of ASD properties. Through such detailed evaluations, selecting the most suitable ASD formulation for further development becomes critical. This selection process is essential to mitigate risks associated with subsequent Chemistry, Manufacturing, and Controls (CMC) studies, clinical trials, and regulatory submissions, thereby streamlining the development pathway and enhancing the likelihood of successful product outcomes.
1. Huang Y, Dai W G. Fundamental aspects of solid dispersion technology for poorly soluble drugs[J]. Acta Pharmaceutica Sinica B, 2014, 4(1): 18-25.
2. Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions[J]. Journal of pharmaceutical sciences, 2012, 101(4): 1355-1377.
3. Alonzo D E, Zhang G G Z, Zhou D, et al. Understanding the behavior of amorphous pharmaceutical systems during dissolution[J]. Pharmaceutical research, 2010, 27: 608-618.
4. 欧丽泉等,聚合物载体对固体分散体稳定性影响的研究进展[J].中国医院药杂志, 2020, 40 (19): 2077-2081.
5. 刘旭等,固体分散体物理稳定性影响因素及抗老化研究进展[J].中国现代应用药学,2011, 28 (8)
6. 吴昊旻等,无定形固体分散体的溶出与吸收研究进展[J]. 药学学报, 2022, 57(5): 1312-1321.
Subscribe to be the first to get the updates!