Leveraging a robust drug formulation services platform, Crystal Pharmatech is recognized as a reliable partner that delivers high-quality, fast, flexible clinical and commercial production services to meet our customers' different needs, helping them accelerate their drug launches.
Dosage forms
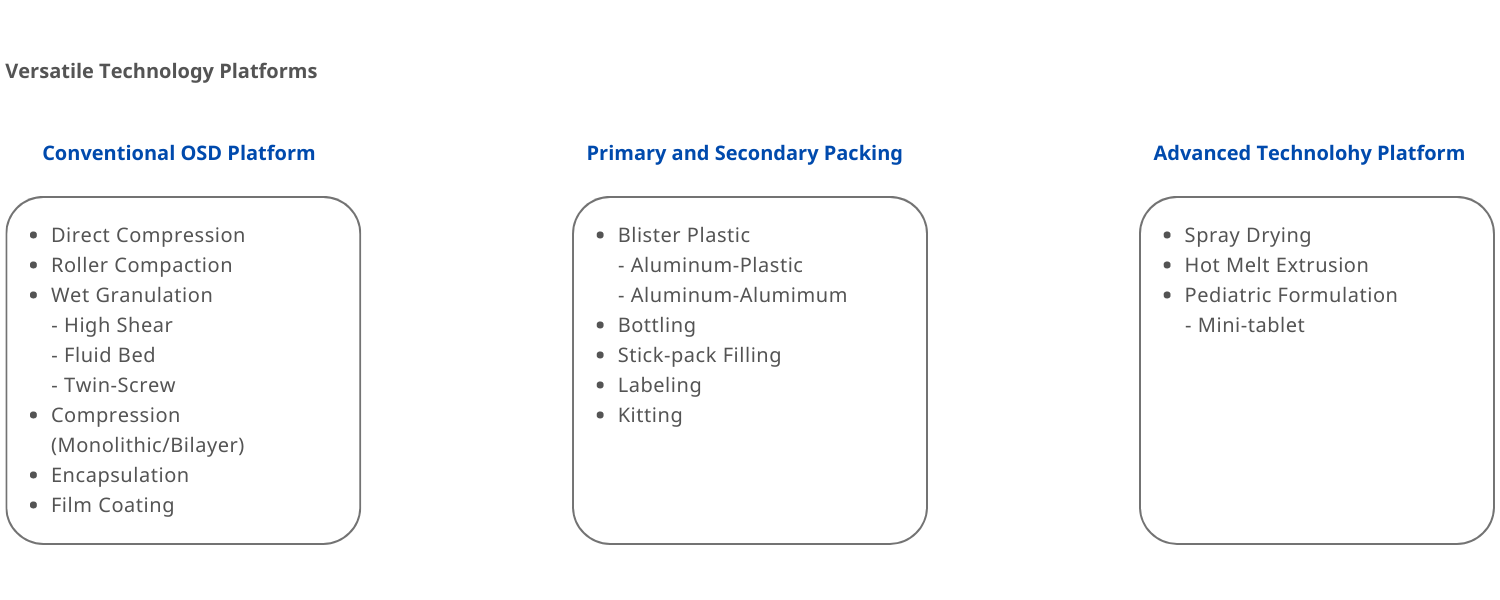
Solid oral dosage forms (tablet, capsule, sachet)
Amorphous Solid Dispersions (ASD, Spray Drying & Hot Melt Extrusion) for insoluble compounds & PROTAC & oral peptides
Pediatric Dosage Form (mini-tablet)
Crystal Pharmatech (CDMO Site in China, Crystal Formulation Services)
located in Suzhou, China, our 50,590 sq. ft. facility is designed to support pharmaceutical and manufacturing needs:
R&D Facility (11,840 sq. ft.) - Dedicated to research and development projects
GMP Facility (17,250 sq. ft.) - Equipped to handle batch sizes up to 120 kg
Clinical Supply Depot (21,500 sq. ft.) - Provides packaging, labeling, storage and distribution, with extended support through a partnered U.S. depot
Crystal Pharmatech (CDMO Site in Canada, Candoo Pharmatech)
located in Toronto, Canada, our 12,500 sq. ft. facilities are designed to meet your pharmaceutical and manufacturing needs:
R&D Facility (3,500 sq. ft.) - Dedicated to research and development projects
GMP Facility (9,000 sq. ft.) - Equipped to handle batch sizes up to 100 kg
An experienced team with highly efficient execution
The GMP team are experts with extensive industry experience, unique skills in optimizing and troubleshooting production and analysis, and broader chemistry expertise. We ensure that your project gets the focus it needs and with our expertise in technical transfer, we can increase manufacturing efficiency.
World-class manufacturing equipment
Taking advantage of our advanced technologies and world-class manufacturing equipment, Cyrstal Pharmatech is uniquely positioned to deliver high-quality drug products to achieve or beyond your goals.
cGMP and QMS meeting NMPA, FDA, and EMA requirements
Crystal Pharmatech’s infrastructure, quality systems, and verification and validation procedures comply with international cGMP standards, laying a solid foundation for us to provide CDMO services that meet the FDA, NMPA, and EMA regulations.

Have a question or need support with your project? Please complete the form, and our team will get back to you shortly.
Our capabilities span three specialized platforms:
Small Molecule
Crystal Bio Solutions
Crystal NAX
By providing your e-mail address, you agree to receive an e-mail response from Crystal Pharmatech to your inquiry. The information you submit will be governed by our Privacy Policy.
Subscribe to be the first to get the updates!