16 May 2024
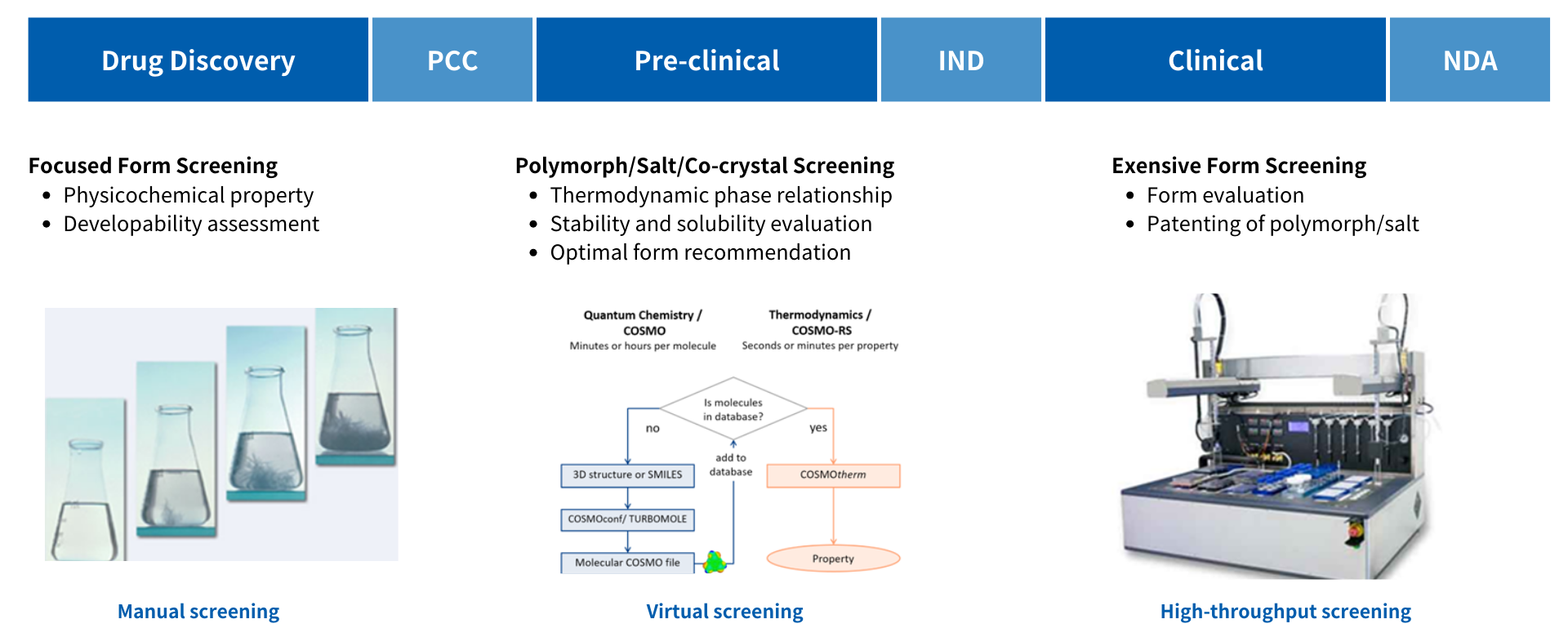
Most solid drugs can exist in different solid forms (polymorph, anhydrate, hydrates, solvates, cocrystals, salts, and the amorphous forms), which lead to different physicochemical properties, such as solubility, stability, particle size and others. Therefore, screening and selection of the ideal API solid forms is critical to obtain the highest standard quality, performance and a good manufactured reproducibly.
Crystal Pharmatech is a world-leading CRO/CDMO specializing in API form and solid-state property research with capabilities to handle potent compounds (OEB 4 & 5) and all categories of controlled substances. What makes us unique is that over the past 13 years, we have been dedicated to solid-state research and CMC services and provide physicochemical properties characterization using XRPD, TGA, DSC, PLM, PSD, etc., form selection (free form/salt/cocrystal), and single crystal growth and structure confirmation for any stage of pharmaceutical development.
Polymorph Screening and Selection
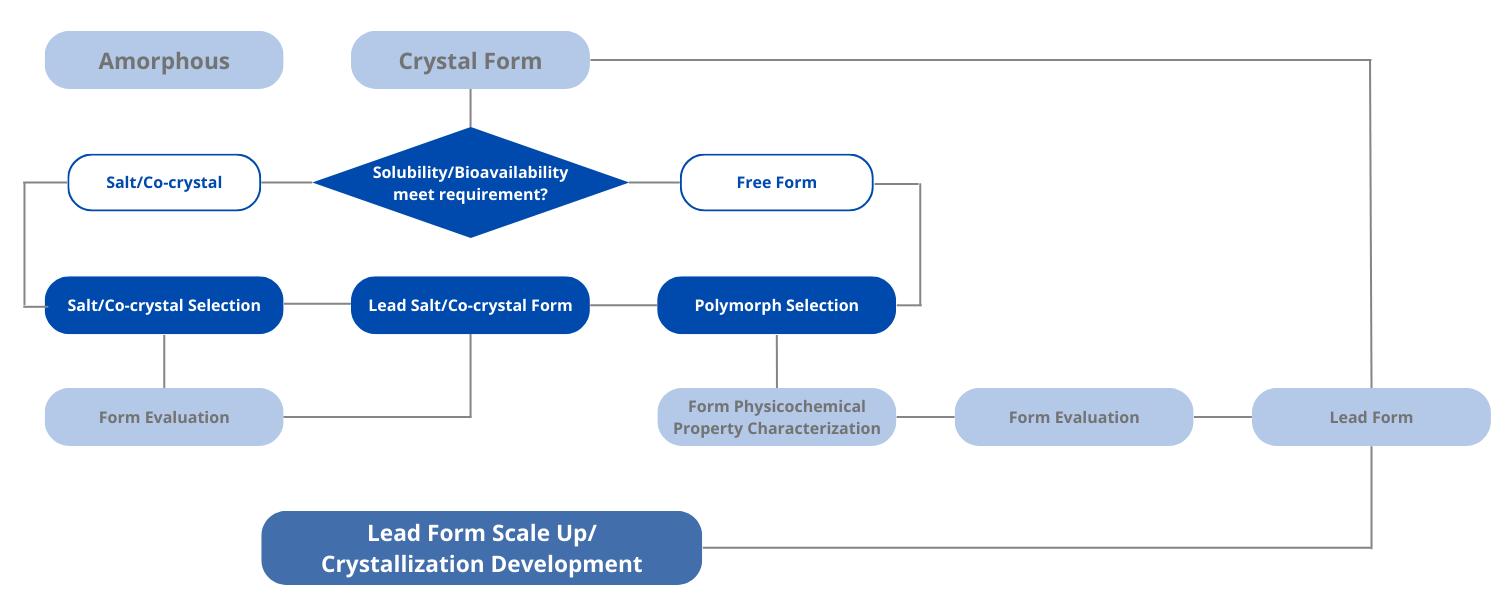
Freeform is given priority for polymorph screening if the physical and chemical properties of the compound can meet the development needs. The ultimate goal of the polymorph screening is to investigate the polymorph landscape of the API molecule and find the thermodynamically most stable polymorph with superior stability, hygroscopicity, and melting point. Our service including:
Design various crystallization conditions according to the physicochemical properties of compound
Crystal form identification and characterization
Thermodynamic phase relationship study
Stability and solubility evaluation
Optimal solid form recommendation
When a compound enters the late clinical stage, to provide comprehensive patent protection for all available crystal forms, innovator companies usually conduct a comprehensive and systematic evaluation of the new drug compound. This type of solid form screening is usually called solid form IP screening. For this stage of screening, the primary goal is to find all crystal forms (usually metastable crystal forms) suitable for industrial development and then seek patent protection for them.
Crystal Pharmatech has established different tiers of crystal form screening strategies and approaches. On the one hand, we will cover a wider range of crystallization methods and solvent systems and select many enabling crystallization methods; on the other hand, we will design experimental conditions that are prone to metastable crystal forms, which are mainly derived from the company's successful experience in supporting hundreds of solid form IP screening.
Salt Screening and Selection
If the stability and bioavailability of the compound need to be improved, the screening of salt and co-crystal will be considered. We offer tailored salt screening and selection to discover the most suitable salt form for development. Our service including:
Design various salt-forming experiments according to the physicochemical properties of compound
Salt form identification and characterization
Thermodynamic phase relationship study
Stability and solubility evaluation
Salt type selection and salt form recommendation
Co-crystal Screening and Selection
Co-crystallization is a crystal-engineering technique employed in case of non-ionizable organic molecules that are unable to form salts. Our approach utilizes the interaction between API and co-formers to choose suitable co-formers and design experiments. Our service including:
Choose suitable co-formers to design experiments
Co-crystal form identification and characterization
Thermodynamic phase relationship study
Stability and solubility evaluation
Co-crystal form recommendation
Capability-Solid Form Screening