Characterization of powder mechanical properties and systematic study of powder compression are critical for guiding formulation and process development, enabling tableting scale-up across product life-cycle management, and supporting troubleshooting. In recent years, these topics have attracted growing attention from both academia and industry. In 2023, the European Pharmacopoeia Commission released draft general chapter <2.9.55>, “Characterization of Powder Behavior During Compression,” and in 2024, the United States Pharmacopeia (USP) published draft general chapter <1245>, “Compaction Simulation.” This article provides a brief introduction to compaction-simulation technology and its applications in the development of oral solid dosage forms.
Powder compression reduces voids in the powder bed (bed porosity) and enhances interparticle bonding. Under applied pressure, particles first rearrange to fill voids; with increasing pressure, they deform and bulk volume decreases. Deformation proceeds via three primary mechanisms—elastic, plastic, and brittle fracture. Elastic deformation is reversible, whereas plastic deformation and brittle fracture are irreversible. Flexible (ductile) materials such as microcrystalline cellulose deform plastically, increasing contact area and contact density. Hard, brittle materials such as lactose undergo brittle fracture, breaking into smaller particles when the local stress exceeds their fracture strength and creating fresh surfaces. Elastic recovery during decompression or ejection can lead to tablet capping, while plastic deformation and brittle fracture create new contact points. These particles then bond through solid bridges, intermolecular forces, and mechanical interlocking, ultimately forming tablets with sufficient mechanical strength.
The relationship between tableting process parameters and tablet quality attributes can be described using the following properties [1][2]:
· Compactibility: the ability of a powder to form tablets of a given strength at a given density, described by the tensile strength–solid fraction curve. It captures the dual effect of pressure on both densification and strength.
· Compressibility: the tendency of a material to reduce volume (increase solid fraction) under pressure, described by the solid fraction–pressure curve. The Heckel and Kawakita models are commonly used to analyze compressibility, infer deformation mechanisms, and estimate yield pressure.
· Tabletability: the ability of a powder to achieve adequate tablet strength as a function of compaction pressure, described by the tensile strength–pressure curve. It reflects how tablet tensile strength increases with applied pressure.
· Manufacturability: a practical analogue to tabletability, tracked by the tablet breaking force–pressure curve.
Tableting pressure is determined by normalizing the applied compression force to the punch-tip area, while tablet tensile strength is derived by normalizing the breaking force to the tablet’s dimensions. Consequently, tabletability (tensile strength–pressure) curves are largely independent of tablet shape and size, making them particularly useful for characterizing mechanical strength and evaluating powder compressibility.
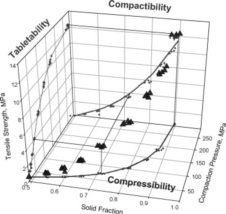
Figure 1 Relationship Between Compactibility, Compressibility, and Tabletability
Compaction simulators have been used in tablet development since the late 20th century and are now established, state-of-the-art tools. Designed to emulate single-punch compaction under rotary-press kinematics, they can reproduce the full compression cycle with as little as ~1 g of material. Equipped with high-precision force/pressure and displacement transducers, they capture punch displacement and pressure profiles throughout the cycle. These instruments are instrumental in studying powder compression behavior, identifying critical material attributes (CMAs) and critical process parameters (CPPs), and establishing their relationships to tablet critical quality attributes (CQAs).
Compaction simulators employ two simulation approaches: direct simulation and theoretical displacement–curve–based simulation. By pressure-generation mechanism, they are further classified as hydraulic or mechanical[3]. The Styl’One, a mechanical simulator using theoretical displacement curves, calculates and reproduces the punch motion—covering pre-compression, main compression, and ejection—by incorporating geometric parameters of the target rotary tablet press (e.g., cam angle, roll diameter, roll spacing) and process parameters (e.g., turret speed). With real-time data acquisition and control, it closely emulates rotary-press kinematics and compaction profiles.
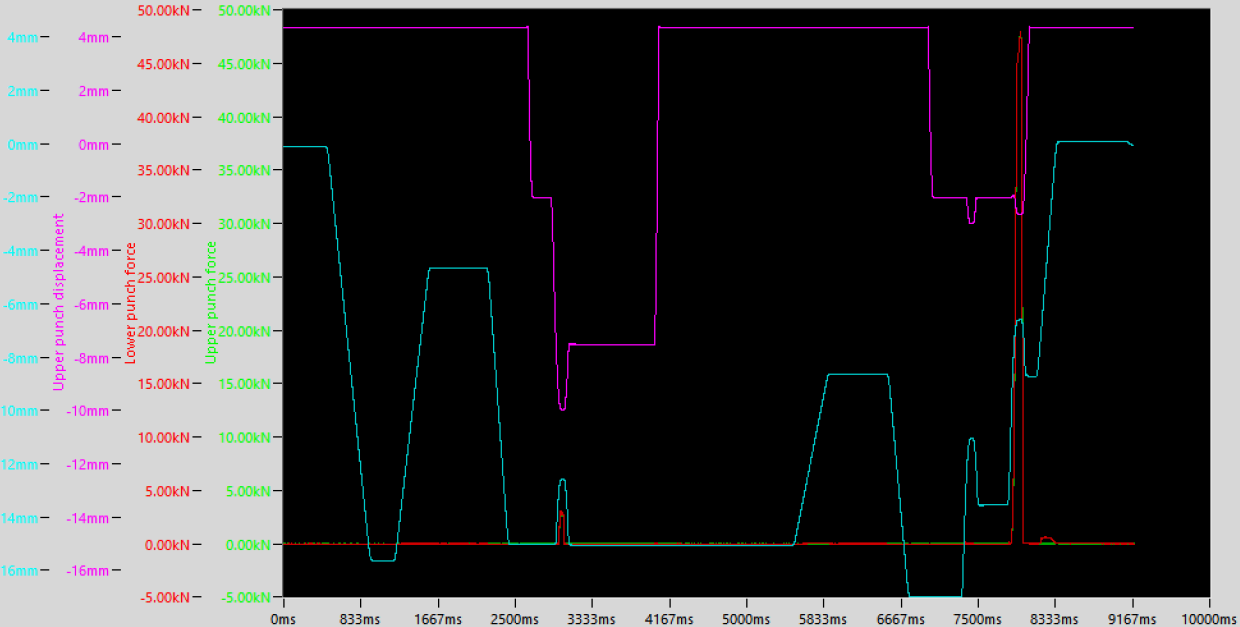
Figure 2 Styl’One Punch Displacement and Pressure Profiles
(1) Formulation and Process Design
Scientific formulation design begins with a rigorous understanding of material properties. The characteristics of active pharmaceutical ingredients (APIs) and excipients directly influence formulation composition and processing choices. For powders with distinct behaviors (e.g., pre-blends vs. final blends), processing conditions must be tailored to ensure that critical quality attributes (CQAs) are achieved. This approach aligns with the Quality by Design (QbD) framework, which emphasizes proactive development to deliver predefined product quality.
Among powder physicochemical properties, compressibility is critical for tablet development. At appropriate compaction pressures, particles bond to yield tablets with sufficient mechanical strength. To ensure robustness through downstream operations (e.g., coating, packaging, transportation), tablet tensile strength is typically targeted at ≥ 1.7 MPa [4]. By contrast, over-compaction can drive porosity too low, leading to delayed disintegration/dissolution, internal microcracks, and an increased risk of capping.
Frictional behavior of powders must also be considered. High die-wall friction can generate excessive ejection force, increasing the risk of edge chipping or capping during tablet ejection. As a guideline, ejection stress should remain below ~3 MPa and never exceed 5 MPa. Ejection-force profiles are also diagnostic for sticking, which typically presents as elevated and fluctuating ejection force.
Characterizing the mechanical properties of APIs, excipients, and intermediates guides manufacturing-process selection and defines the design space for tablet formulations and compaction parameters. This proactive approach helps mitigate risks such as high friability, capping, and sticking during scale-up and commercial production. For high-dose APIs with poor compressibility, the formulation’s overall compressibility is largely governed by the API’s inherent properties; in such cases, dry granulation may be suboptimal. To minimize batch-to-batch variability, drug loading should be optimized and excipients chosen judiciously to balance powder mechanics. When dry granulation is employed, compaction-simulation data on the pre-blend can guide roller-pressure settings to produce granules with suitable porosity, particle-size distribution, bulk/tapped density, and flowability. Similarly, simulation data for the final blend inform the selection of main-compression forces to balance tablet strength with disintegration and dissolution performance.
(2) Scale-Up of Tableting Process
During laboratory development, single-punch (single-station) presses are typically used for tableting, whereas rotary tablet presses are employed at pilot and commercial scale. Because rotary presses require substantially more material, they are generally unsuitable for early-stage work. At high turret speeds, rotary-press dwell time is markedly shorter than on laboratory single-punch presses. Depending on a material’s strain-rate sensitivity (SRS), this difference introduces scale-up risk: tablet mechanical properties may deteriorate (e.g., reduced tensile strength or increased friability), with a higher incidence of capping or lamination and potential failure to meet dissolution specifications.

The Styl’One compaction simulator replicates rotary tablet press operation via theoretical displacement curves. During laboratory development, it can emulate high-speed production conditions with gram-scale material, enabling early assessment of scale-up effects and potential risks on tablet performance. In doing so, it both guides formulation/process design and supports subsequent tableting scale-up. By contrast, compression studies on rotary presses typically require kilogram-scale (or larger) quantities to satisfy equipment and process demands. Figure 3 compares Styl’One simulations (under identical material and punch-tooling conditions) with compression data from a Killian KTP 420X rotary press[5]; Styl’One closely approximates the KTP 420X, demonstrating high fidelity to real-world compaction behavior.
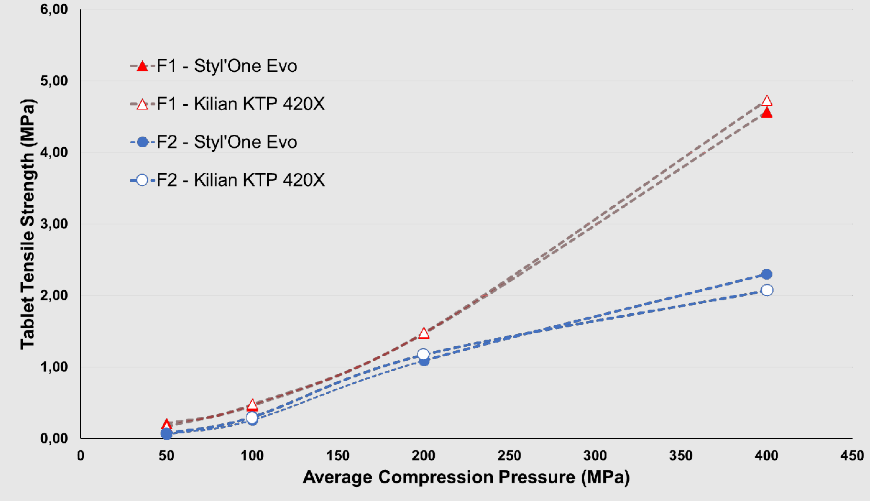
Figure 3 Comparison of Styl’One-Simulated and Actual Compression Results for the Target Rotary Tablet Press
Figure 4 compares Styl’One-simulated results across different rotary presses at varying speeds under identical material and punch-tooling conditions. For a Korsch XL100 at 20 rpm, the dwell time is ~130 ms; for a Korsch XL400 at 40 rpm, the dwell time is ~14 ms. Tablets compressed with shorter dwell times (e.g., XL400) exhibit lower tensile strength at equivalent compaction pressures. Accordingly, to achieve comparable strength on the XL400, higher compaction pressures are required—consistent with strain-rate effects.
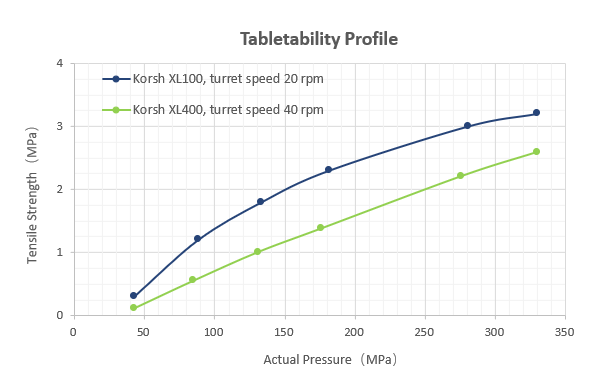
Figure 4 Comparison of Styl’One-Simulated Results for Different Rotary Press Models at Varying Compression Speeds
Excessively high compression speeds in commercial production are a major cause of capping. Compaction simulators enable early identification of the critical compression speed (at a given pressure) beyond which capping occurs. Pre-compression—typically set at 5–20% of the main-compression force—substantially mitigates capping risk, and simulators facilitate optimization of pre-compression settings.
In summary, prior to tableting process scale-up, compaction simulators allow systematic study of CPPs and their effects on tablet CQAs using minimal material, supporting the establishment of appropriate process-parameter ranges for scale-up.
(3) Troubleshooting
Common challenges in tableting include capping, lamination, sticking, excessive ejection force, and failure to meet specifications for hardness, friability, disintegration time, weight variation, or content uniformity. Issues such as sticking often surface only during scale-up. Troubleshooting directly on rotary presses is time- and material-intensive, whereas compaction simulators enable rapid, small-scale studies to identify root causes and screen solutions, which are subsequently confirmed on rotary equipment.
Ultimately, tableting problems originate from material properties. Compaction simulators characterize mechanical behavior, improving understanding of how material attributes and process parameters interact to determine product quality. This scientific approach enables efficient, targeted resolution of complex challenges.
The application of compaction simulation technology enhances the fundamental understanding of powder compression principles and mechanical properties, establishing scientific insights into the interplay among material attributes, processes, and product quality. By enabling data-driven formulation and process design, this technology ensures robust product quality. As a versatile tool, the compaction simulator plays a pivotal role in formulation development, tableting process scale-up, and troubleshooting, demonstrating significant potential for broader industrial adoption.
Compaction simulation technology is integral to solid dosage form development at Crystal Formulation Service (CFS). Leveraging extensive project experience, we have developed predictive models to scientifically guide formulation and process design, forecast post-compression product properties, and reduce development timelines and material demands. These advancements empower us to enhance product quality, improve efficiency, and minimize trial-and-error costs.
[1] Yihong Qiu, Yisheng Chen, Geoff G.Z. Zhang, et al. Developing solid oral dosage forms [M]. 1st ed. Burlington: Academic Press, 2009.
[2] Ching Kim Tye, Changquan (Calvin) Sun, Gregory E. Amindon. Evaluation of the Effects of Tableting Speed on the Relationships between Compaction Pressure, Tablet Tensile Strength, and Tablet Solid Fraction [J]. Journal of pharmaceutical science, 2005, 94(3): 465-472.
[3] Nenad Nikolić. Usage of compaction simulators for the powder compression characterization – advantages and limitations [J]. Arh. Farm, 2022, 72: 546-565.
[4] Medpharm. Characterization guideline Excipients/API/Formulations.
[5] University of Dortmund, Germany -APV Expert Workshop Tableting –April 2017.
Subscribe to be the first to get the updates!