In 2012, drug development specialists started an initiative to create predictive Oral Bioavailability Tools (OrBiTo). This initiative recognized the need to move away from random trial-and-error in vivo drug development by integrating more efficient in vitro and in silico methods.
At the end of 2022, President Joe Biden signed legislation formalizing this shift away from heavy in vivo reliance. New medications can now be approved by the U.S. Food and Drug Administration (FDA) without animal studies.
While it is recognized that animal studies will still play an important role in new drug research and approvals for the foreseeable future, this new legislation signals an industry-wide shift towards in silico studies that must be acknowledged.
Drug innovators looking for the most effective and efficient drug development pathway to bring promising molecules to market need to give careful consideration to the approach they choose.
Wasteful, prolonged trial-and-error methodologies for form and formulation are a thing of the past. Today's industry standard requires that drug developers guide development through a strategic approach, from lead compound optimization to first in human (FIH) clinical trials.
| "Smart preclinical development using established innovative tools saves time, money, and lives." |
| - Robert Wenslow, Ph.D., Co-Founder and VP of Business Development |
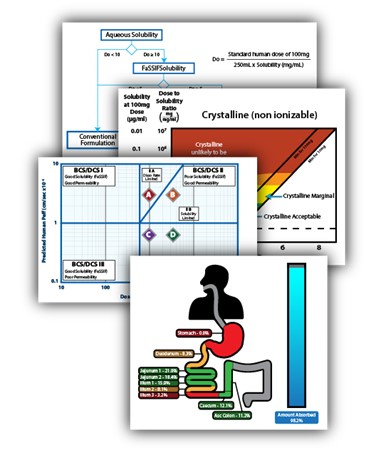
Crystal Pharmatech moves molecule to medicine by adopting a constantly evolving approach that brings together cutting-edge AI capabilities, the most efficient in vitro platforms, and leading experts in API form and formulation.
The result of this approach is the revolutionary Mol2Med™ Developability Assessment, putting an end to outdated and inefficient guesswork.
The Mol2Med™ Developability Assessment is a dynamic, fit-for-purpose service that helps drug developers achieve a comprehensive understanding of their molecule’s potential for development.
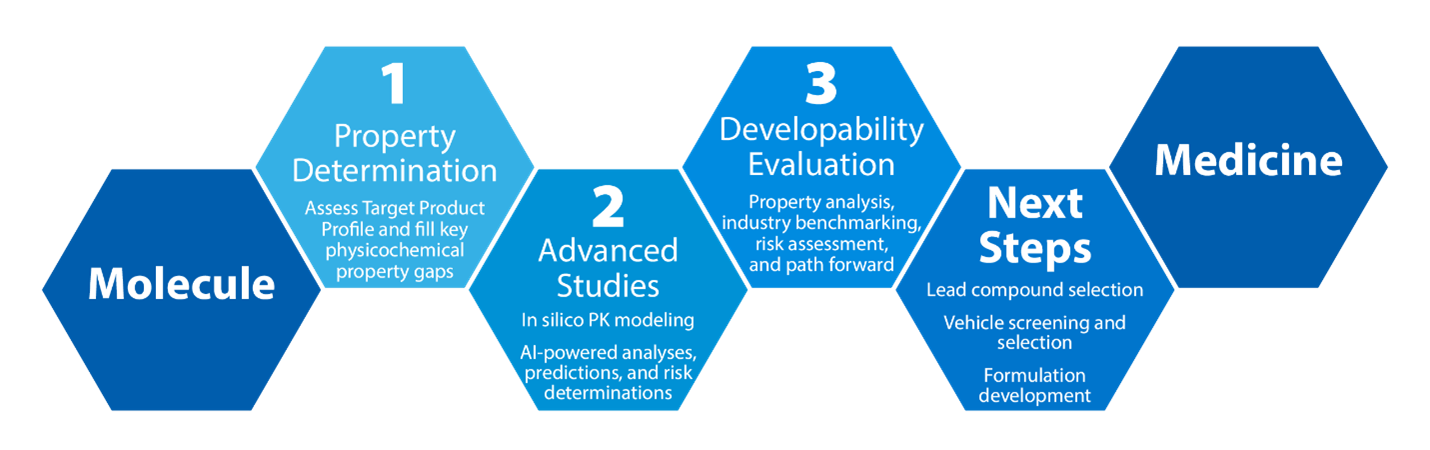
Mol2Med™ Developability Assessment Workflow
With as little as 500mg of API and 4 weeks, Crystal Pharmatech offers a smarter path forward by:
Determining the key physicochemical properties of up to 7 compounds
Generating AI-powered insights
Benchmarking compound(s) according to industry standards
Providing a high-level approach for achieving most probable success in GLP Tox and FIH
Crystal Pharmatech R&D and Subject Matter Experts have decades of experience moving molecules to medicine.
The Mol2Med™ pathway offers specific next steps to reach all major milestones throughout drug development program - from lead compound selection to formulation development.
Crystal Pharmatech experts provide clear insights that help drug innovators:
Expedite development timeline
Minimize costs through a strategic scope of work
Maximize a molecule's likelihood of success in GLP Tox and FIH
Leading experts will ensure CMC is not limiting Tox and Phase I success while assessing later stage developability risks of API and drug product.
With cohesion between solid-state and formulation experts, drug innovators partnering with Crystal Pharmatech gain continuity and a goal-oriented approach throughout every stage of development.
For further information, follow and stay updated at the Crystal Pharmatech LinkedIn page.
Subscribe to be the first to get the updates!