As the demand for small molecule drugs with high bioavailability continues to grow, the development of hydrophobic new chemical entities that have very low water solubility also increasing. Recent statistics indicate that approximately 70% of small molecules under development belong to Biopharmaceutics Classification System (BCS) II or IV poorly water-soluble drugs.
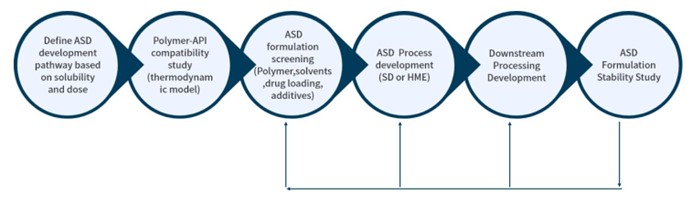
Crystal Pharmatech utilizes advanced amorphous solid dispersion (ASD) technology to help our clients solve this low solubility issue. Our two platforms include spray drying and hot melt extrusion. Both spray drying and hot melt extrusion methods can be utilized to prepare ASD, enhancing solubility and oral bioavailability.
Spray Drying
The spray drying process is a particle engineering technique that is a proven approach to improving the solubility of poorly soluble compounds. It has been widely used to enhance bioavailability in drug manufacturing for the following advantages:

Scalable and cost-effective technique
Consistent particle size control
Ability to handle temperature-sensitive molecule
Long-term stability
The core technical team at Crystal Pharmatech has solid knowledge and expertise in the fundamentals and scaling-up principles for spray drying process and commercialized several amorphous solid dispersion solid dosage form drug products. With our deep understanding of all quality and regulatory requirements and world-class manufacturing and analytical equipment, we are confident that we can support amorphous solid dispersion formulation development at high speed and with high quality.
Hot Melt Extrusion
HME is widely recognized as an effective technology that enhances the bioavailability of poorly soluble APIs, ultimately improving patient compliance and making the manufacturing process more efficient.

Can be operated as a continuous process, ensuring optimal reproducibility. Appropriate for large-scale production.
Less offline testing is required, and online PAT can be easily implemented.
A limited number of processing steps, short residence time, and reduction in labor forces lead to higher economic efficiency.
Solvent-free process, environmentally friendly.
Relatively high drug load possible.
The core team of Crystal Pharmatech has a strong background in polymer materials, chemical industry, and machinery, adhering to the guidance of rheology and engineering principles, and has deep attainments in the field of HME. We have developed formulations and technology platforms including immediate release and sustained release and have a successful experience in process scale-up and commercial production. Like spray drying, we have world-class bench-to-production process equipment and analytical instruments. We hope to help innovative drug customers overcome the barriers of insoluble drugs with HME's method.
Have a question or need support with your project? Please complete the form, and our team will get back to you shortly.
Our capabilities span three specialized platforms:
Small Molecule
Crystal Bio Solutions
Crystal NAX
By providing your e-mail address, you agree to receive an e-mail response from Crystal Pharmatech to your inquiry. The information you submit will be governed by our Privacy Policy.
Subscribe to be the first to get the updates!