From early developability to late-stage specifications—polymorph/salt-cocrystal strategy, SCXRD/MicroED solutions, solvent & pathway design, drug-product form control, ASD crystalline-form limits, PROTAC readiness, and crystal-form IP support.
Small-molecule APIs often exist in multiple solid forms—polymorphs, salts, cocrystals, solvates/hydrates, and amorphous phases. Insufficient early understanding of form exposes development to avoidable risks across CMC, manufacturability, intellectual property, and clinical performance, risks that are amplified by compressed timelines and limited API supply. A systematic, hypothesis-driven solid-form screening program mitigates these risks by: (i) development—mapping the polymorphic landscape, establishing relative stabilities (including solvates/hydrates), and generating solubility and stability data to define a robust crystallization route; (ii) regulatory—building a defensible rationale and control strategy aligned with ICH guidance and expectations of the Chinese Pharmacopoeia, NMPA, FDA, and EMA; and (iii) patent—discovering as many forms as practicable, evaluating novelty and inventive step, and compiling enabling evidence for crystal-form patents. The result is confident selection and justification of a lead crystal form, a reduced likelihood of late-stage form changes, a well-defined operating space for scale-up and tech transfer, and stronger IP and regulatory dossiers.
Polymorph development typically follows a “screen-to-select” workflow: first collect solid-state characterization data on the starting material; then, guided by solubility behavior, design polymorph screens across diverse solvents and processing conditions; classify any new forms (anhydrate, hydrate, or solvate); establish interconversion relationships among anhydrous forms and between hydrates/solvates and anhydrates; and finally compile decision data for the preferred form, including hygroscopicity, stability, and solubility.
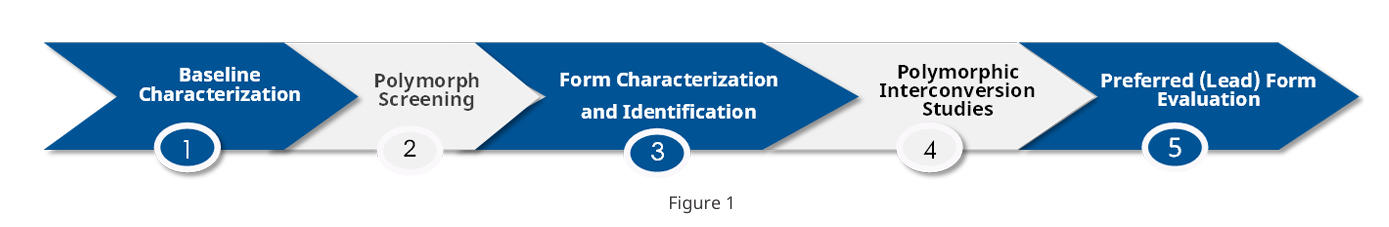
For patent-focused polymorph screening, work is typically staged and may involve 200–300—or more—screening experiments. The design of each subsequent phase is informed by results from the preceding phase.
Table 1.
Stage | Methods | No. of experiments |
Stage 1 | Antisolvent addition | 100 |
Vapor–liquid diffusion | ||
Vapor–solid diffusion | ||
Slow evaporation | ||
Slow cooling | ||
Slurry equilibration (stirred) | ||
Temperature cycling (heating–cooling) | ||
Stage 2 | Reverse antisolvent addition (good solvent into antisolvent) | 100 |
Slurry equilibration (stirred) | ||
Rapid evaporation | ||
Quench/rapid cooling | ||
Polymer-induced crystallization | ||
Grinding (neat or liquid-assisted) |
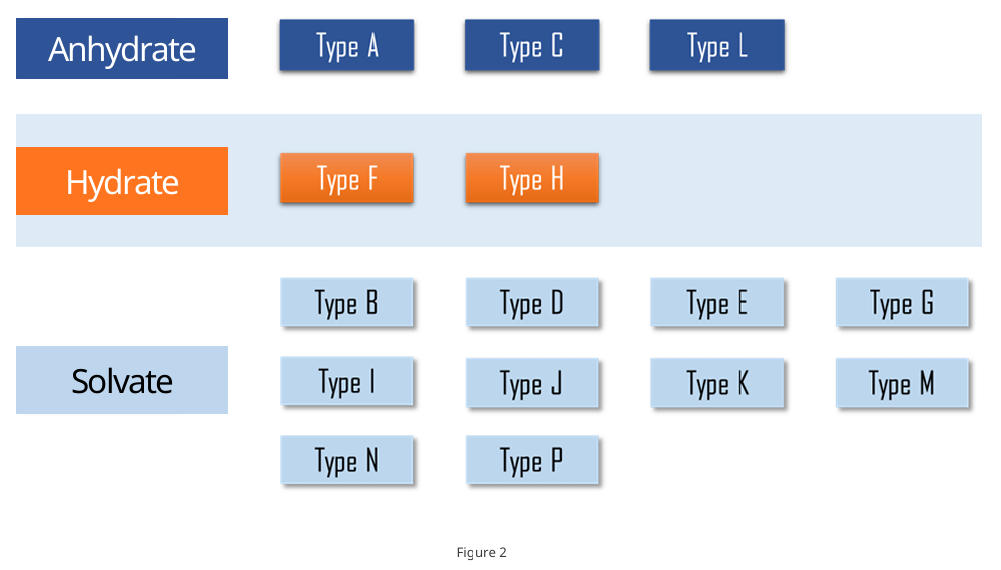
For any new forms discovered during screening, qualitatively assign the form class (anhydrate, hydrate, or solvate). For hydrates and anhydrates, conduct follow-on studies to establish and evaluate interconversion relationships.
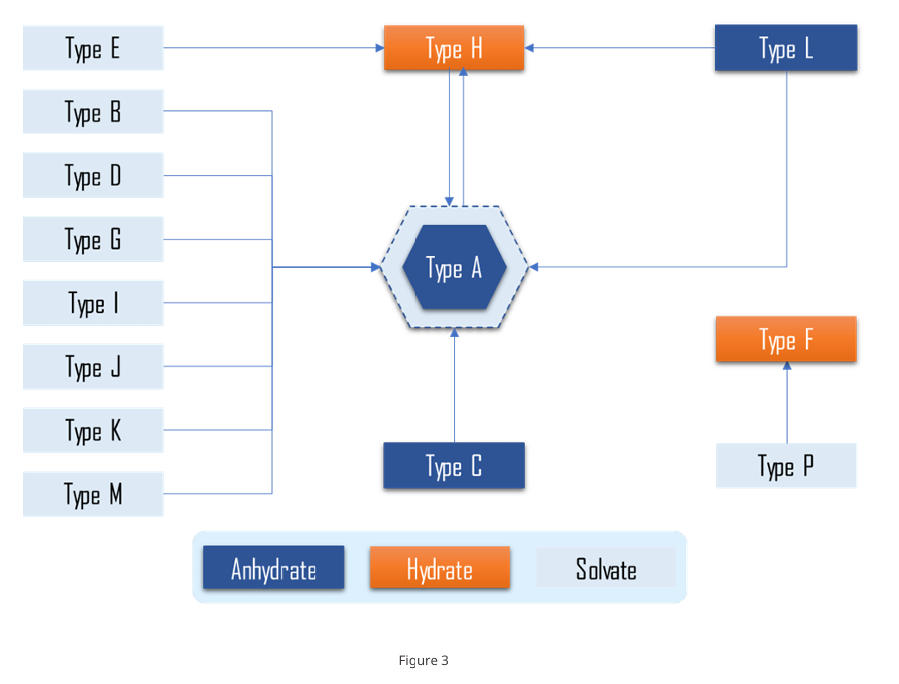
For anhydrous polymorphs, determine the thermodynamic stability relationships and identify the thermodynamically most stable anhydrate. For anhydrate–hydrate and hydrate–hydrate pairs, define the humidity/water-activity (RH, Aw) conditions that govern interconversion.
Form pair | Methods |
Anhydrate ↔ Anhydrate | Slurry competition (non-aqueous solvent systems) |
Burger–Ramberger rule (DSC) | |
van ’t Hoff plot (solubility) | |
Anhydrate ↔ Hydrate | Slurry competition (solvent systems at different water activities) |
Humidity-induced transformation (DVS / saturated salt solutions) | |
Hydrate ↔ Hydrate | Slurry competition (solvent systems at different water activities) |
Humidity-induced transformation (DVS / saturated salt solutions) |
Compile decision-enabling data for the preferred crystal form—hygroscopicity, stability, and solubility—to guide formulation design and drug-product development. For crystal-form patent protection, broaden the work to mine additional, differentiating evidence across multiple dimensions to substantiate novelty and inventive step.
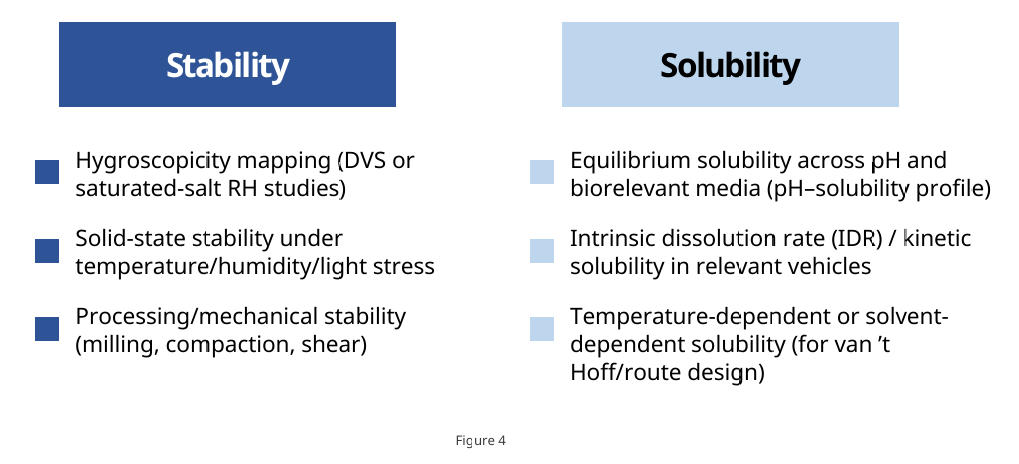
Stability
· Hygroscopicity mapping (DVS or saturated-salt RH studies)
· Solid-state stability under temperature/humidity/light stress
· Processing/mechanical stability (milling, compaction, shear)
Solubility
· Equilibrium solubility across pH and biorelevant media (pH–solubility profile)
· Intrinsic dissolution rate (IDR) / kinetic solubility in relevant vehicles
· Temperature-dependent or solvent-dependent solubility (for van ’t Hoff/route design)
Solid-form studies span the entire drug-development lifecycle. Establishing an early understanding of available forms, identifying the key drivers of form interconversion, and applying targeted studies and controls throughout development can markedly reduce late-stage rework and supplemental investigations—lowering effort, cost, and overall development risk.
Crystal Pharmatech, founded in 2010, is a global contract research organization with approximately 300 employees and four R&D centers in New Jersey (USA), San Francisco (USA), Toronto (Canada), and Suzhou (China). Collectively, these sites provide integrated support for pharmaceutical and biotechnology companies worldwide. Our capabilities span three specialized platforms: in small molecules, we offer API solid state research and crystallization, preformulation, formulation development, and GMP manufacturing and supply; under Crystal Bio Solutions, we deliver bioanalytical and biomarker testing, biologics CMC analytics, and clinical pharmacology; and through Crystal NAX (Nucleic Acid Excellence), we provide end-to-end solutions for nucleic acid therapeutics, from early research through clinical development.
Subscribe to be the first to get the updates!