Salt formation is a well-established strategy for improving the physicochemical properties of active pharmaceutical ingredients (APIs) containing ionizable groups. This approach is particularly effective in enhancing aqueous solubility, often leading to improved bioavailability for poorly soluble compounds¹. Beyond solubility, salt formation can also provide advantages in process development and stability. Crystalline salts, for example, are generally easier to isolate and purify than amorphous free forms, while conversion to a more stable salt form can mitigate issues related to chemical instability. It is estimated that nearly half of all marketed APIs are formulated as salts². Nevertheless, salt selection and development require careful consideration of potential liabilities, including the risk of disproportionation, counterion-related toxicity, and increased drug loading requirements³.
To provide a deeper understanding of salt disproportionation, this article examines the underlying reaction equilibria, key influencing factors, and potential mitigation strategies. The discussion focuses on salts formed between basic APIs and acids, which account for the majority of marketed salt forms⁴. However, the same fundamental principles apply to salts derived from acidic APIs and basic counterions.
Salt disproportionation describes the transformation of a highly soluble salt form into its poorly soluble, non-ionic free base or free acid. This process is governed by a series of equilibria that define both the formation of the salt and its dissolution behavior under varying pH conditions.
· Salt Formation Equilibrium
For basic APIs, the salt formation equilibrium can be represented as:
![]()
The equilibrium constant (K) for this reaction is determined by the dissociation constants of the free base (Kab) and the acid (Kaa), expressed as:
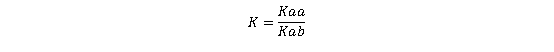
(Equation 1)
· Dissolution Equilibrium of Salt and Free Base
The dissolution equation of the salt is given by:
![]()
The solubility of the salt (Ss) can be described by its solubility product (Ksp), ignoring common ion effects for simplicity:
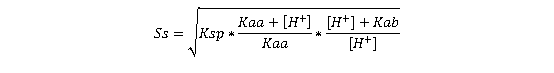
(Equation 2)
The dissolution equilibrium of the free base is given by:
![]()
The solubility of the free base (Sb) is expressed in terms of its intrinsic solubility (S₀, equivalent to [B]):
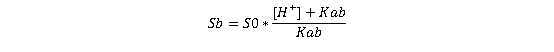
(Equation 3)
By plotting Ss (Equation 2) and Sb (Equation 3) as functions of pH (i.e., [ H+] ), two solubility curves are obtained, as illustrated in Figure 1.
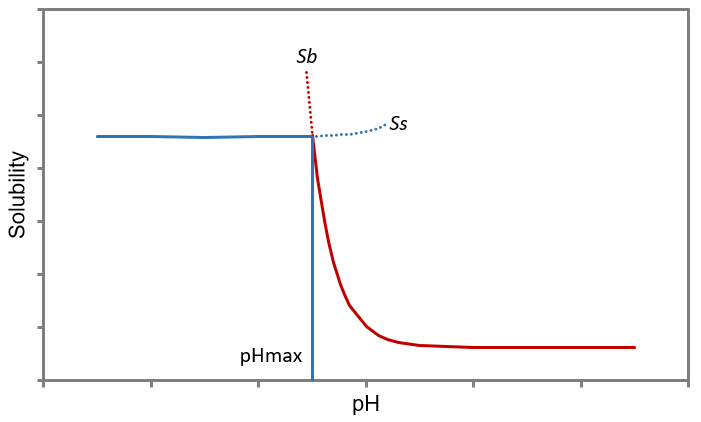
Figure 1 Schematic diagram of solubility curves of basic APIs versus pH
At the intersection of these curves, the salt and the free base coexist in equilibrium, where Ss equals Sb. Under the condition where Kaa≫[H+]≫Kab, the hydrogen ion concentration at this intersection point is:
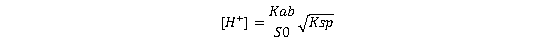
This corresponds to a pH value known as pHmax⁵, defined by:
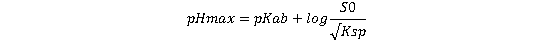
(Equation 4)
1) Impact of pKaa and pKab
According to the equilibrium constant in Equation (1), a lower Kab (corresponding to a higher pKab) favors salt formation and reduces the risk of disproportionation. Literature surveys of approved salt-based drug products indicate that no marketed examples possess a pKab below 4.6. Consequently, it is generally recommended to prioritize candidates with a pKab greater than 5.0 for salt development⁶. Molecules with pKab values below this threshold should be considered with caution and evaluated on a case-by-case basis.
Additionally, based on the equilibrium defined in Equation (1), maximizing salt formation requires a sufficiently large ratio of Kaa to Kab. A commonly accepted guideline is that the difference in pKa values (ΔpKa) should be at least 3 units. Salt formation is generally considered favorable when the pKab of the basic API exceeds the pKaa of the counterion acid by 3 or more units⁷.
2) Solubility Considerations
As shown in Figure 1, for basic APIs, the total API concentration in solution remains relatively stable as pH increases—assuming pH is the only influencing factor and common ion effects are negligible. However, beyond a certain pH threshold, the concentration begins to decline. This critical point is defined as pHₘₐₓ, and it plays a pivotal role in predicting the physical stability of the system⁶. When the system pH exceeds pHₘₐₓ, the solubility of the salt surpasses that of the free base, leading to precipitation of the free base and the onset of disproportionation. A higher pHₘₐₓ is generally favorable, as it extends the pH range over which the salt remains stable and reduces the risk of disproportionation.
As indicated by Equation (4), a higher pKab results in an elevated pHₘₐₓ, which is advantageous for minimizing the risk of salt disproportionation—consistent with the preceding analysis. Achieving a higher pHₘₐₓ also depends on the relationship between the intrinsic solubility (S₀) of the free base and the solubility product (Ksp) of the salt. Specifically, S₀ should not be too low relative to Ksp, implying that salts with excessively high Ksp values (i.e., high solubility) may be more susceptible to disproportionation. Although enhanced solubility is often a key objective during salt screening to improve drug absorption, excessive solubility can potentially compromise physical stability. Therefore, when several salt candidates meet the desired solubility threshold for formulation, preference should be given to those with comparatively lower Ksp values. Such salts are inherently more resistant to disproportionation and may offer improved stability during development⁶.
3) Microenvironment pH Control
Once a specific salt form of an API is selected, its corresponding pHₘₐₓ is defined. To minimize the risk of disproportionation during formulation development, it is critical to control the pH of the excipient microenvironment. When the local pH remains below pHₘₐₓ, the salt form is stable. However, if the microenvironment pH exceeds pHₘₐₓ, the likelihood of disproportionation increases⁶.
For salts derived from basic APIs, excipients with alkaline properties are particularly problematic, as they can elevate the microenvironment pH and promote conversion of the salt to the free base. Thus, excipients that contribute to a more acidic local environment are generally preferred. In cases where the use of high-pH excipients is unavoidable, incorporation of suitable pH modifiers may be necessary to maintain salt stability. Measuring the microenvironment pH in solid dosage forms remains technically challenging. Two commonly used approaches include: 1) Suspending the formulation in water and measuring the pH of the resulting saturated solution; 2) Mixing the formulation with a pH-sensitive indicator and determining the ionization state via diffuse reflectance spectroscopy⁸.
4) Humidity and Moisture Management
Salt disproportionation is a solution-mediated process. Even in solid-state formulations, it is generally accepted that disproportionation can occur rapidly within localized aqueous microenvironments. Elevated humidity and moisture content promote this by enabling transient hydration at the particle level. Several studies have shown that high humidity exposure can trigger or accelerate disproportionation⁹˒¹⁰. While a strong quantitative correlation is not always observed, high water content is broadly recognized as a contributing factor¹¹. To mitigate this risk, moisture exposure should be minimized during formulation and processing. Dry granulation is often preferred over wet granulation to avoid introducing water. For packaging, moisture-barrier materials and desiccants can limit water uptake. Hygroscopic excipients should also be avoided to reduce local moisture accumulation and the risk of disproportionation.
5) Temperature Effects
Temperature influences both the thermodynamics and kinetics of salt disproportionation. In general, higher temperatures increase the likelihood of disproportionation occurring¹². To reduce this risk, salt-form APIs and their formulations should be stored under controlled, lower-temperature conditions whenever feasible.
· Disproportionation of Salts with Different Aqueous Solubility
Thakral et al.¹³ investigated the disproportionation behavior of three CRH-1 salt forms: hydrochloride, hydrobromide, and hemi-1,5-napadisylate. Among these, the hydrochloride exhibited the highest solubility, while the hemi-1,5-napadisylate showed the lowest, with differences attributable in part to their distinct stoichiometries (Table 1). Tablets were formulated using each salt in combination with excipients, and their propensity for disproportionation was evaluated. Under accelerated conditions (40 °C/75% RH/open), the hemi-1,5-napadisylate remained stable over an extended duration, whereas the hydrochloride displayed greater disproportionation than the hydrobromide. The corresponding Ksp values and pHₘₐₓ data are summarized in Table 1 and shown in Figure 2. The hydrochloride, characterized by higher solubility (Ksp) and lower pHₘₐₓ, was more susceptible to disproportionation—potentially exacerbated by hydrogen chloride volatility. In contrast, the hemi-1,5-napadisylate, with lower Ksp and higher pHₘₐₓ, exhibited improved resistance to disproportionation.
Table 1 Ksp and pHₘₐₓ of Different Salts of CRH-1
| Salt | pHmax | Ksp |
| HCl | 1 | 2.8 x 10-3 [M]2 |
| HBr | 1.3 | 7.7 x 10-4 [M]2 |
| Hemi-1,5-napadisylate | 2.5 | 8.0 x 10-10 [M]3 |
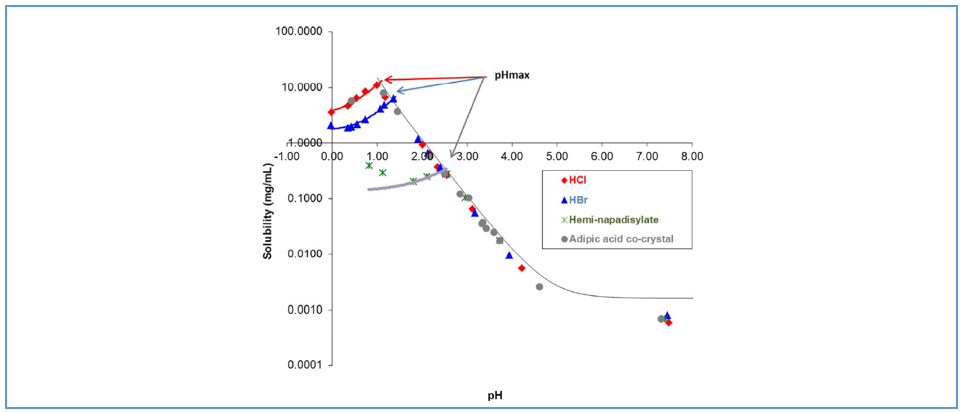
Figure 2 Solubility curves of hydrochloride, hydrobromide, and hemi-1,5-napadisylate versus pH (based on experimental data)
· The Impact of pH Modifiers on Salt Disproportionation
Koranne et al.¹⁴ studied the impact of various organic acid pH modifiers on the disproportionation behavior of pioglitazone hydrochloride (PioHCl). Magnesium stearate and a range of organic acids (Table 2) were incorporated as excipients. Powder blends of the API and excipients were prepared and stored under accelerated conditions (40 °C/75% RH). The extent of free base formation was assessed using X-ray powder diffraction (XRPD), with results presented in Figure 3. The control formulation, lacking any acidifier, showed rapid disproportionation followed by a plateau. In contrast, formulations containing pH modifiers exhibited reduced levels of free base, indicating suppression of disproportionation. Notably, no disproportionation was observed in formulations containing maleic acid or oxalic acid. Comparison with the pKₐ values of the acids listed in Table 2 suggests that stronger acids are more effective at mitigating disproportionation in PioHCl systems.
Table 2: Organic acids used as pH modifiers
Organic acid | Chemical structure | pKa (pKa1, pKa2) | Aqueous solubility (mol/L), 25 °C |
Oxalic acid/OA |
| 1.2, 4.2 | 1.56 |
Maleic acid/MA |
| 1.9, 6.2 | 4.05 |
Tartaric acid/TA |
| 3.0, 4.3 | 8.66 |
Fumaric acid/FA |
| 3.0, 4.3 | 0.05 |
Glutaric acid/GA |
| 4.3, 5.4 | 4.77 |
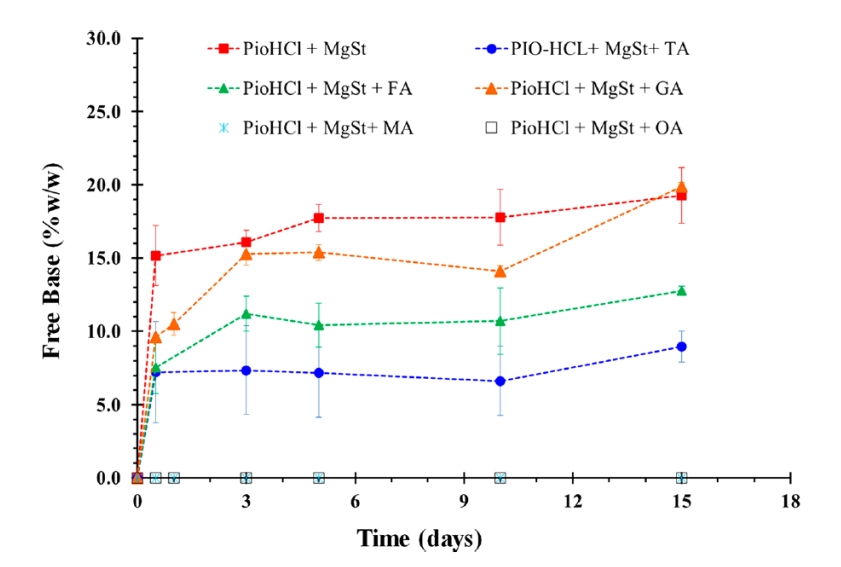
Figure 3: Content of pioglitazone free base over time for different systems after storage at 40 °C/75% RH (mean ± SD; n=3)
· Impact of Ambient Relative Humidity and Moisture Content on Disproportionation
Weldeab et al.³ examined the effect of excipients with varying water content—trisodium phosphate dodecahydrate (TSPD) and anhydrous trisodium phosphate (TSP)—on the disproportionation behavior of Compound 1 tartrate salt. Binary mixtures of the API with each excipient were prepared and stored under both open and closed conditions to evaluate the extent of disproportionation. As shown in Figure 4, for a given type and amount of excipient, disproportionation occurred more readily under open conditions at 40 °C/75% RH compared to closed conditions at 40 °C, highlighting the role of ambient humidity in promoting salt instability. Figure 5 further demonstrates that at an excipient-to-API molar ratio of 0.36:1, the TSPD-containing system—due to its higher water content—exhibited greater disproportionation (i.e., increased free base formation) than the system with anhydrous TSP. These findings confirm that formulations with higher moisture content are more susceptible to salt disproportionation.
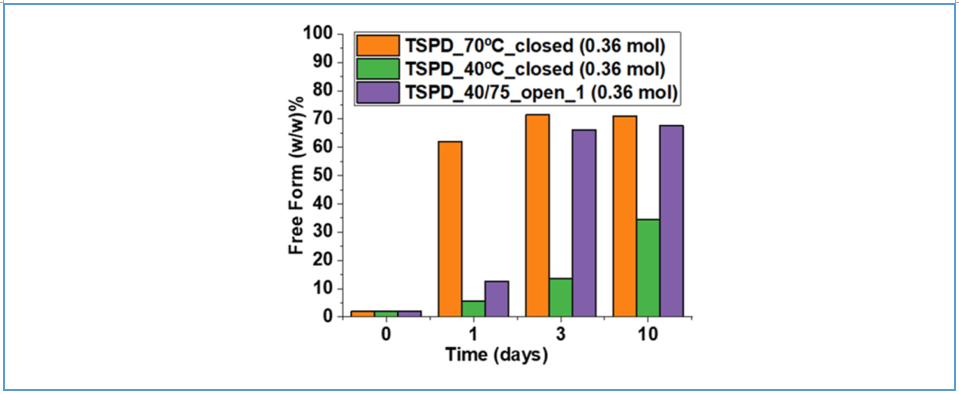
Figure 4: Bar graph showing the extent of disproportionation for Compound 1 tartrate + TSPD mixtures at different time points under various conditions
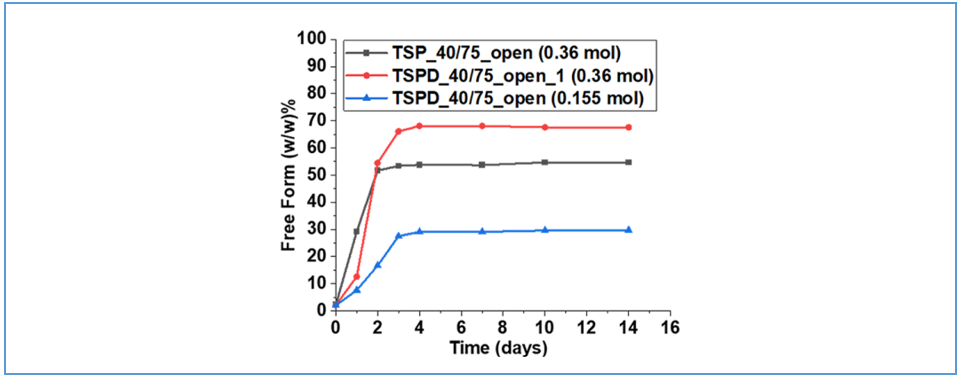
Figure 5: Disproportionation curves for Compound 1 tartrate + TSP or TSPD mixtures at different time points under various conditions
Effective mitigation of API salt disproportionation requires a comprehensive understanding of both the intrinsic properties of the salt and the characteristics of its surrounding environment.
From the perspective of intrinsic salt properties, basic APIs should possess a high pKab, ideally exceeding the pKaa of the counterion acid by at least three units to favor salt formation and stability. Additionally, the selected salt should exhibit appropriate solubility; when bioavailability requirements are met, salts with comparatively lower solubility are generally preferred due to their reduced susceptibility to disproportionation. From an environmental perspective, minimizing the risk of disproportionation involves selecting excipients that maintain a lower microenvironmental pH, avoiding exposure to elevated temperature and humidity during storage, and carefully controlling the moisture content within the formulation.
Crystal Formulation Services (CFS) provides clients with comprehensive salt-form screening and formulation evaluation services, delivering integrated solutions and end-to-end support throughout drug development. At the salt screening stage, the Crystal Pharmatech technical team—leveraging over a decade of practical experience—conducts a systematic and scientifically rigorous screening process to identify the most suitable salt form for further development. During formulation development, we conduct a thorough assessment of salt disproportionation risk and apply a data-driven approach to excipient selection, formulation design, and process optimization. When the potential for disproportionation is identified, targeted controls—such as tailored packaging strategies and storage condition recommendations—are implemented based on stability data. In cases where disproportionation occurs in solid dosage forms, the appearance of the free base is typically detectable via distinct X-ray powder diffraction (XRPD) patterns. Our specialized instrumentation and expertise in solid-state characterization ensure effective monitoring and evaluation of such transformations, supporting robust and stable drug product development.
[1] Amidon, G.L., Lennernäs, H., Shah, V.P., Crison, J.R., A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability, Pharm. Res., 1995, 12, 413-420, -Backstory of BCS. AAPS J. 2014, 16, 894−898.
[2] Saal, C., Becker, A., Pharmaceutical salts: A summary on doses of salt formers from the Orange Book, Eur. J. Pharm. Sci., 2013, 49, 614−623.
[3] Weldeab, A.O., McElderry, J.D., Lin, Y., The Effect of In-Situ-Generated Moisture on Disproportionation of Pharmaceutical Salt, Mol. Pharmaceutics, 2023, 20, 1, 561–571.
[4] Serajuddin, A.T., Salt formation to improve drug solubility, Adv. Drug Deliv., 2007, Rev. 59, 603-616.
[5] Thakral, N.K., Kelly, R.C, Salt Disproportionation: A Material Science Perspective, Int. J. Pharm., 2017, 520 (1−2), 228−240.
[6] Stephenson, G.A., Aburub, A., Woods, T.A., Physical Stability of Salts of Weak Bases in the Solid-State, J. Pharm. Sci., 2011, 100, 1607-1617.
[7] Bastin, R.J., Bowker, M.J., Slater, B.J. Salt selection and optimisation procedures for pharmaceutical new chemical entities, Org. Process Res. Dev., 2000, 4, 427-435.
[8] Govindarajan, R., Zinchuk, A., Hancock, B., Shalaev, E., Suryanarayanan, R. Ionization states in the microenvironment of solid dosage forms: effect of formulation variables and processing, Pharm. Res., 2006, 23, 2454-2468.
[9] Hsieh, Y.L., Taylor, L.S. Salt stability-effect of particle size, relative humidity, temperature and composition on salt to free base conversion, Pharm. Res., 2015, 32, 549-561.
[10] Merritt, J.M., Viswanath, S.K., Stephenson, G.A., Implementing quality by design in pharmaceutical salt selection: a modeling approach to understanding disproportionation, Pharm. Res., 2013, 30, 203-217.
[11] Patel, M.A., Luthra, S., Shamblin, S.L., Arora, K., Krzyzaniak, J., Taylor, L.S., Effect of excipient properties, water activity, and water content on the disproportionation of a pharmaceutical salt, Int. J. Pharm., 2018, 546, 226−234.
[12] Christensen, N.P.A., Rantanen, J., Cornett, C., Taylor, L.S., Disproportionation of the calcium salt of atorvastatin in the presence of acidic excipients, Eur. J. Pharm. Biopharm., 2012, 82, 410-416.
[13] Thakral, N. K., Behme, R. J., Aburub, A., Peterson, J. A., Woods, T. A., Diseroad, B. A., Suryanarayanan, R., Stephenson, G. A., Salt Disproportionation in the Solid State: Role of Solubility and Counterion Volatility. Mol. Pharmaceutics, 2016, 13 (12), 4141–4151.
[14] Koranne, S., Lalge, R., Suryanarayanan, R., Modulation of Microenvironmental Acidity: A Strategy to Mitigate Salt Disproportionation in Drug Product Environment. Mol. Pharmaceutics, 2020, 17 (4), 1324−1334.
Subscribe to be the first to get the updates!