News
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
04 Dec 2025
 In-Silico PBPK Modeling and Simulation
Crystal Pharmatech now provides Physiologically Based Pharmacokinetics (PBPK) simulation capability.
In-Silico PBPK Modeling and Simulation
Crystal Pharmatech now provides Physiologically Based Pharmacokinetics (PBPK) simulation capability.
 Excipient Selection and Compatibility Studies
Excipients play an important role in drug release, drug stability and manufacture of drug product. We help customers select the appropriate excipients in early formulation development through screenin...
Excipient Selection and Compatibility Studies
Excipients play an important role in drug release, drug stability and manufacture of drug product. We help customers select the appropriate excipients in early formulation development through screenin...
 Analytical Chemistry
We will provide our valued customers with analytical chemistry research and quality control services for NCEs throughout their life cycle from the early clinical stage to commercial manufacturing. Ana...
Analytical Chemistry
We will provide our valued customers with analytical chemistry research and quality control services for NCEs throughout their life cycle from the early clinical stage to commercial manufacturing. Ana...
 Flow Cytometry
Cell therapy and biomarker (e.g. immunophenotyping and receptor occupancy assays)
Flow Cytometry
Cell therapy and biomarker (e.g. immunophenotyping and receptor occupancy assays)
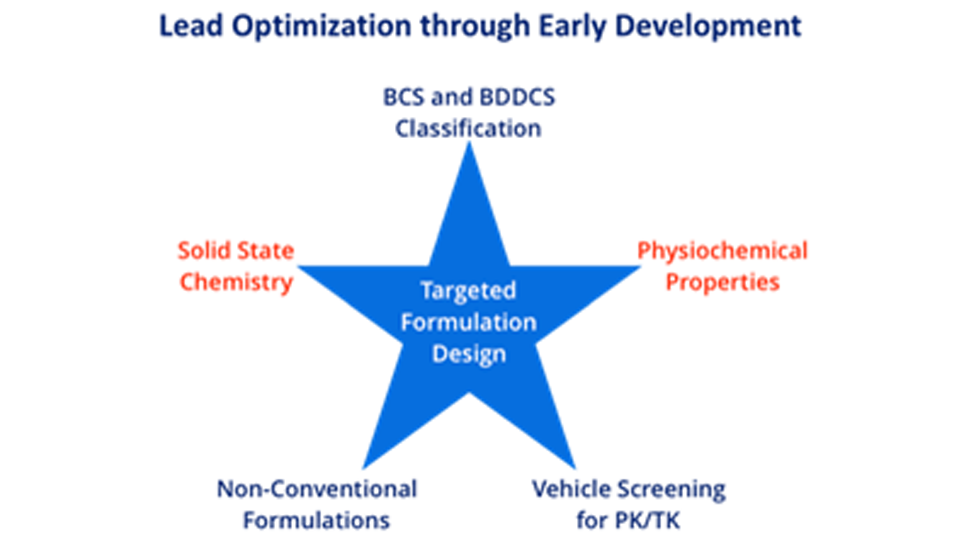 Formulations for PK/Efficacy/Tox Studies
We adopt integrated approach to find the right form and formulation through our SMART services, leading to the shortest possible development timelines with limited resource utilization.
Formulations for PK/Efficacy/Tox Studies
We adopt integrated approach to find the right form and formulation through our SMART services, leading to the shortest possible development timelines with limited resource utilization.
 ELISpot Assays
Vaccine efficacy and characterization of cell-mediated immune response
ELISpot Assays
Vaccine efficacy and characterization of cell-mediated immune response
 Solid-State NMR Analysis
Nuclear Magnetic Resonance (NMR) Spectroscopy is a ubiquitous analytical technique utilized across a wide range of disciplines.
Solid-State NMR Analysis
Nuclear Magnetic Resonance (NMR) Spectroscopy is a ubiquitous analytical technique utilized across a wide range of disciplines.
 Animal Dosing Vehicle Selection
Choosing an optimal vehicle for animal PK and Tox studies requires multidisciplinary skillsets and a sound understanding of the physicochemical properties of your compound. Controlling solid and solut...
Animal Dosing Vehicle Selection
Choosing an optimal vehicle for animal PK and Tox studies requires multidisciplinary skillsets and a sound understanding of the physicochemical properties of your compound. Controlling solid and solut...
 Molecular Biology
Cell/gene therapy, RNA vaccines, gene editing therapy and biomarkers utilizing qPCR, ddPCR, RNA-Seq, NanoString, and NGS
Molecular Biology
Cell/gene therapy, RNA vaccines, gene editing therapy and biomarkers utilizing qPCR, ddPCR, RNA-Seq, NanoString, and NGS
 Clinical and Commercial Manufacturing
We provide flexible and reliable OSD manufacturing services using versatile technologies to address your formulation challenges.
Clinical and Commercial Manufacturing
We provide flexible and reliable OSD manufacturing services using versatile technologies to address your formulation challenges.
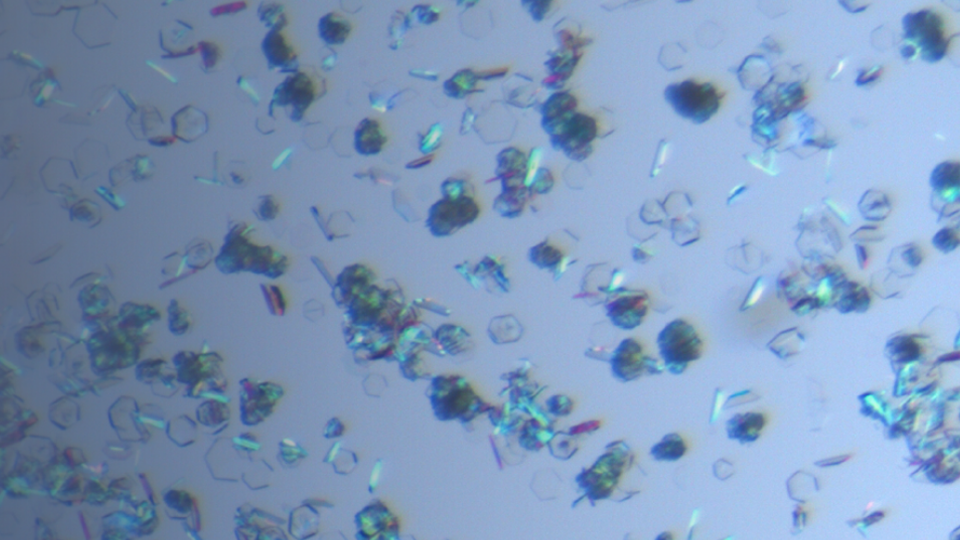 Amorphous Solid Dispersions
Amorphous solid dispersions have been shown to alter the properties of an API, including solubility, dissolution, and bioavailability. A variety of polymers or additives are available, and screening i...
Amorphous Solid Dispersions
Amorphous solid dispersions have been shown to alter the properties of an API, including solubility, dissolution, and bioavailability. A variety of polymers or additives are available, and screening i...
 FIH-PMF-FMF Manufacturing
First-in-human (FIH) formulation development is done for phase I-IIa CTM Manufacturing to advance FIH clinical trial and obtain proof of concept (POC) of the safety and efficacy of the molecule under ...
FIH-PMF-FMF Manufacturing
First-in-human (FIH) formulation development is done for phase I-IIa CTM Manufacturing to advance FIH clinical trial and obtain proof of concept (POC) of the safety and efficacy of the molecule under ...
 Clinical Supply
Crystal Pharmatech provides a one-stop Clinical Supply Service from pre-clinical to post-marketing. Services provided: Primary packagingcGMP environment; cGMP requirements; Blistering, bottling, capsulat...
Clinical Supply
Crystal Pharmatech provides a one-stop Clinical Supply Service from pre-clinical to post-marketing. Services provided: Primary packagingcGMP environment; cGMP requirements; Blistering, bottling, capsulat...
Subscribe to be the first to get the updates!