Amorphous solid dispersion (ASD) is a formulation strategy in which a drug is molecularly dispersed in a suitable polymer matrix, forming an amorphous solid solution or suspension that can markedly enhance apparent solubility and oral bioavailability. However, the amorphous state sits at a higher free-energy level than the crystalline form, offering higher solubility but lower thermodynamic stability and a natural tendency to recrystallize. Given the potentially large solubility gap between amorphous and crystalline phases, it is critical to assess ASD physical stability and control crystallization so that phase transformation does not erode dissolution performance or bioavailability.
In the following case study, we describe the strategy used to develop and control the amorphous solid dispersion of everolimus, with a focus on crystalline-phase quantitation and limit-setting.
Everolimus tablets contain the API in an amorphous form, which provides significantly higher solubility than the crystalline state. According to patent disclosures, amorphous everolimus exhibits approximately a 25-fold increase in solubility compared with its crystalline counterpart. However, amorphous everolimus readily crystallizes when slurried in common organic solvents. To address this, the originator formulated everolimus as an amorphous solid dispersion (ASD) and incorporated 0.2% BHT (an antioxidant) to enhance the physicochemical stability of the amorphous API.
Crystal-form content refers to the proportion of a specific crystalline form present in a test sample, typically expressed as a percentage. Crystal-form content analysis encompasses quantitative or limit tests for that crystalline component in the product.
Common techniques include X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), infrared spectroscopy (IR), Raman spectroscopy (RM), solid-state NMR (ss-NMR), and dynamic vapor sorption (DVS), among others.
Key considerations include: setting appropriate limits/specifications; selection of primary standards; preparation and qualification of reference materials; establishing the quantitative principle (linking the chosen physical response to crystal-form content); method validation; ensuring sample representativeness; and method transferability.
Multiple high-resolution XRPD instruments from different manufacturers and models are used to develop and validate qualitative and quantitative methods for crystal-form analysis in both API and drug product, perform routine sample testing, and support method transfer across laboratories or sites.
|  |
| Rigaku SmartLab 9kW | PANalytical Empyrean |
|  |
| PANalytical X'Pert3 | Bruker D8 Discover |
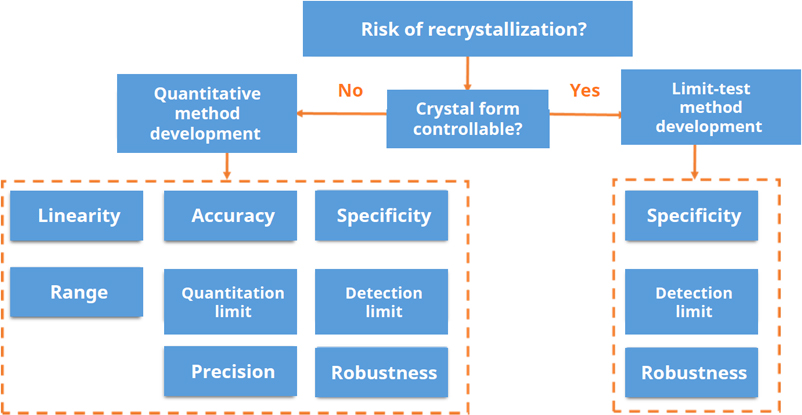
In general, if formation of a crystalline impurity form can be avoided by optimizing the manufacturing and formulation process, or if highly sensitive qualitative tests at an early stage show no detectable impurity form, development of a limit test is usually sufficient. By contrast, for certain generic products where the API has been confirmed to exist as a mixture of crystal forms, or where the reference product has already established a fully quantitative method, a quantitative assay is recommended for crystal-form quality control.
Using everolimus tablets as an example, for both new drugs and generics in which the API is present in an amorphous state (either as an ASD intermediate or by directly formulating the amorphous API), crystal form needs to be controlled at all key stages to ensure that the crystalline API remains below the limit of detection, thereby maintaining the dissolution performance and bioavailability required for clinical use. Stability samples should likewise be monitored periodically for recrystallization to confirm shelf-life stability. Consequently, impurity-form limit studies should be conducted for both the ASD intermediate and the finished dosage form, and in some cases also for the amorphous API itself.
When defining detection/limit levels for these studies, it is advisable to consider the extent of amorphous-to-crystalline conversion observed in process and stability studies, as well as the impact of the impurity form on dissolution and bioavailability. For generic products, the reference product and related formulations can also be reviewed to provide a useful benchmark.
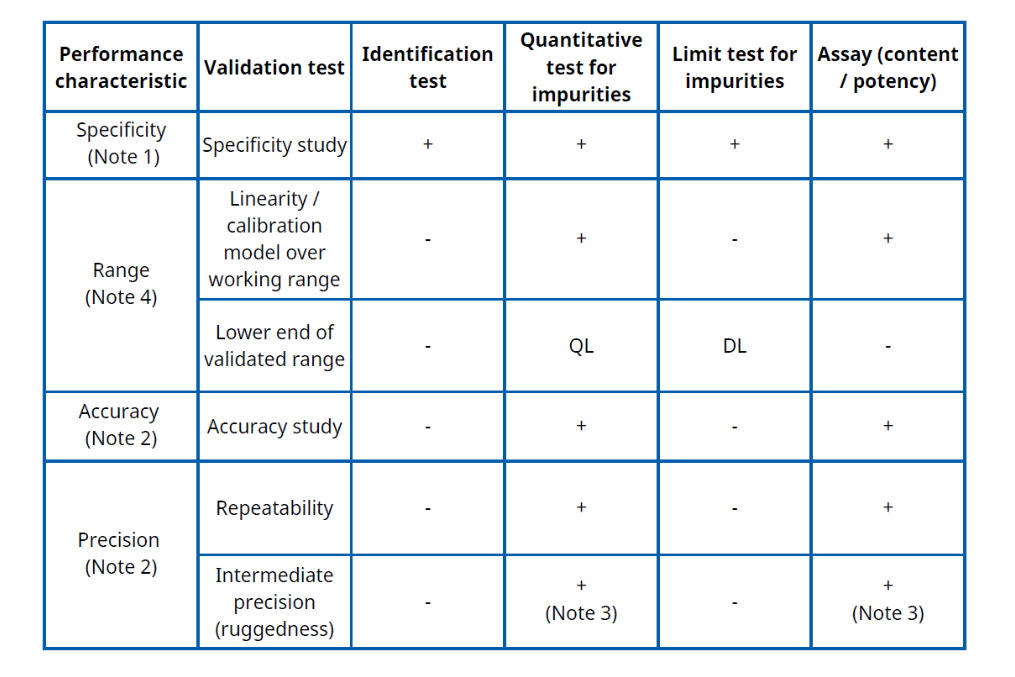
Table.1. FDA. ICH Q2(R2) Validation of Analytical Procedures guidance
Notes
1. Specificity must be demonstrated for all four procedure types; if one method is not fully specific, supporting/orthogonal procedures are expected.
2. Accuracy and precision are required for quantitative procedures (impurity quantitation and assay), but are not normally evaluated for identification tests or simple limit tests.
3. If reproducibility has already been established in a multi-laboratory study, a separate intermediate-precision study is not strictly required (the FDA ICH Q2 text gives this flexibility).
4. For impurity quantitation, the validated range typically extends from the reporting level / QL up to about 120% of the impurity specification; for limit tests, suitability is usually demonstrated at or around the DL; for assay, range is normally about 80–120% of the test concentration.
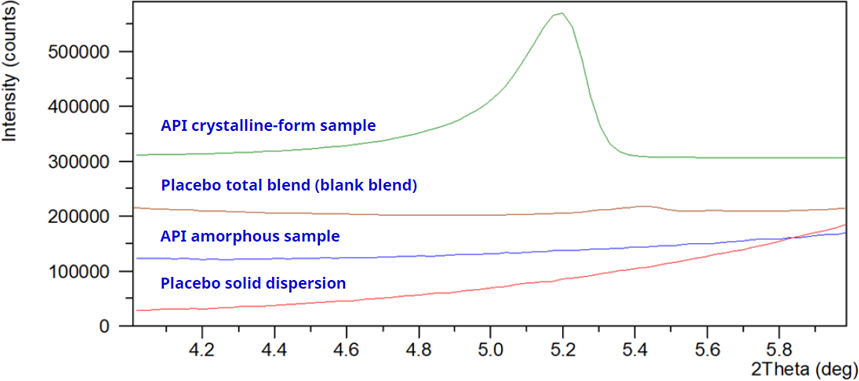
Figure 6.
Characteristic-peak approach: select diffraction peaks from the crystalline impurity form whose positions are not impacted by signals from the amorphous API, the ASD intermediate, or placebo excipients, and use these non-overlapping peaks as the marker peaks for developing the limit test method.
LOD is typically verified using the signal-to-noise (S/N) approach: the LOD standard is analyzed six times with the developed method, using the baseline near the characteristic peak as the noise. For each run, the S/N ratio and the RSD of the peak response are calculated. The criterion s/n > 3 with RSD < 20% is taken as evidence that the LOD verification is acceptable.
Table 2
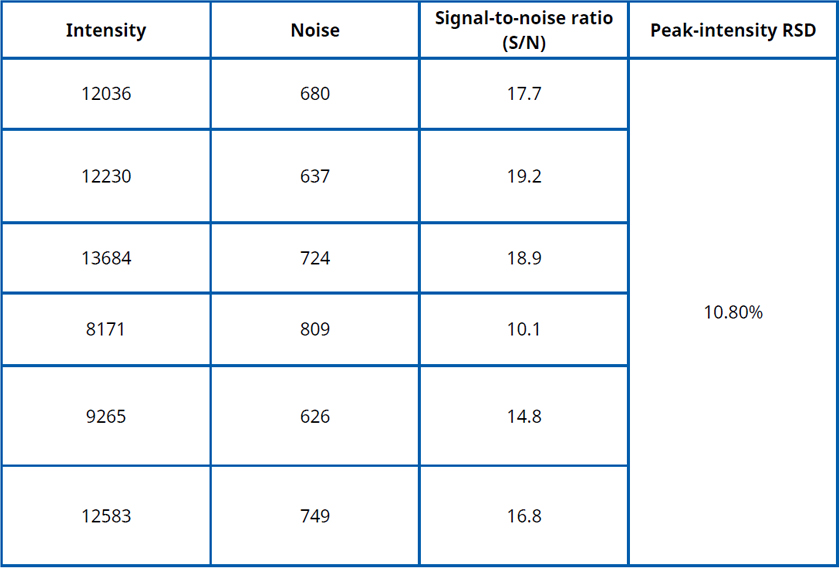
To assess robustness, deliberately vary a selected method parameter and test the LOD standard. If the resulting signal-to-noise ratio remains > 3, the method is considered to have passed the robustness evaluation.
By reviewing patent literature and considering the formulation process, storage conditions, and stability study design, the recrystallization pathways of the amorphous API in the ASD intermediate and finished product can be identified, along with suitable XRPD characteristic peaks for quantitative method development. Using high-resolution XRPD, a specific and sensitive quantitative (limit) method can be established, achieving a detection limit of approximately 0.5% w/w of the ASD intermediate or final dosage form, or lower. Applying this validated XRPD method to the ASD intermediate, drug product, and stability samples, the crystalline impurity should be controlled below the limit of detection, thereby demonstrating that no recrystallization of the amorphous API occurs during manufacture, storage, or stability testing.
Reference
1. CH Q2(R2) Validation of Analytical Procedures: Guidance for Industry. U.S. Food and Drug Administration, December 2022.
Subscribe to be the first to get the updates!