As amorphous solid dispersions (ASDs) and their formulations advance from lab-scale research to clinical trials and, ultimately, to commercial production, manufacturing scales increase from grams to kilograms or even tons. During this scale-up, critical powder properties—such as particle size distribution, flowability, compressibility, and residual solvent content—can change, potentially impacting key tablet performance attributes, including hardness and dissolution rate.
Therefore, establishing robust process parameters and determining appropriate settings for critical conditions—such as drying chamber inlet/outlet temperatures, condensation temperature, gas flow rate, feeding speed, and atomization parameters—is essential when operating across different equipment scales.
The inlet temperature provides the thermal energy necessary for product drying. If set too low, solvent removal may be incomplete, leading to particle agglomeration or wall-sticking, which reduces yield and alters critical powder properties. Conversely, excessively high inlet temperatures can cause degradation of heat-sensitive components. Effective drying of the atomized feed while preserving desired quality attributes requires that the inlet temperature be optimized in relation to material characteristics and other drying parameters.
For example, a 10% w/w solution of mannitol and trehalose (9:1) was spray dried and examined by scanning electron microscopy (SEM) to access particle morphology (Figure 1). At an inlet temperature of approximately 110 °C, smooth, spherical particles were obtained. Increasing the inlet temperature accelerated the evaporation rate, yielding particles with progressively rougher surfaces [2].
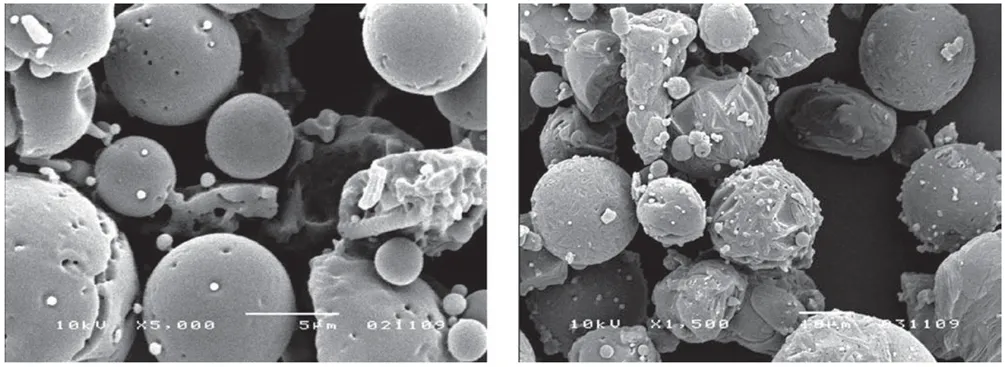
Figure 1. SEM Images of Spray-Dried Particles
(Left: Inlet temperature 110 °C; Right: 220 °C)
The atomizer plays a pivotal role in both drying efficiency and particle characteristics, as it disperses the liquid feed into fine droplets. A uniform droplet size distribution enhances drying performance and improves product quality. Under otherwise identical drying conditions, higher atomization pressures generate finer droplets, which in turn produce smaller particle sizes.
Several atomizer types are commonly used in spray drying:
· Rotary Atomizer: Generates droplets by centrifugal force using a rapidly spinning disc or wheel. Produces particles typically ranging from 20 to 300 μm and is well suited for high-viscosity liquids.
· Two-Fluid Nozzle: Uses a high-velocity gas stream to atomize the liquid into fine droplets, typically 10 to 100 μm in size. Ideal for low-viscosity liquids and thermally sensitive materials. This is the most common type used in small-scale spray drying.
· Pressure Nozzle: Forces the liquid through a narrow orifice under high pressure, forming droplets with particle sizes usually between 50 and 300 μm. Frequently preferred in scale-up processes to improve powder performance.
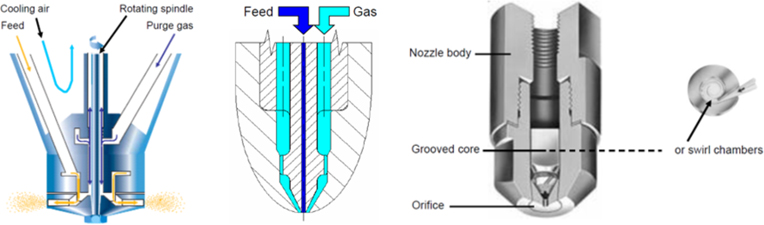
Figure 2. Schematic of Atomization Nozzles
(Left: Rotary atomizer; Middle: Two-fluid nozzle; Right: Pressure nozzle)
As an example, mixed solutions of hydroxypropyl methylcellulose phthalate (HPMCP) and hydroxypropyl methylcellulose (HPMC) at various concentrations and ratios were spray dried using a two-fluid nozzle. Feed rate and outlet temperature had limited impact on particle size. However, reducing atomization pressure produced larger droplets, which in turn increased the particle size of the dried powder [3].
.jpg)
Figure 3. Relationship Between Droplet Size and Particle Size (Dn50)
Feeding speed directly affects both drying efficiency and particle properties. If set too high, atomization efficiency decreases, generating larger droplets that may not fully dry, leading to elevated residual solvent levels.
On the other hand, an excessively low feeding speed reduces the thermal load required for evaporation and can promote solute precipitation on the atomizer, potentially causing nozzle blockage. In scale-up operations, low feeding speed can also substantially extend processing time and increase operational costs.
Unlike the inlet temperature, the outlet temperature reflects the cumulative thermal effect of the entire drying process. It is determined by inlet temperature, feeding speed, gas flow rate, and other operational parameters. Measured at the discharge end of the drying chamber – where most of the solvent has been removed and the product is in a dry state – the outlet temperature directly influences final product properties such as particle morphology, residual solvent, and stability.
To prevent thermal degradation, the outlet temperature is generally maintained below the maximum thermal tolerance of the product. In many cases, keeping it near the boiling point of the solvent also facilitates efficient drying.
For example, when spray drying a 14% w/w HPMCP solution in methanol/water (8:2), outlet temperatures exceeding methanol’s boiling point (65 °C) caused the internal pressure within drying particles to surpass the external pressure, resulting in particle expansion and formation of smooth, spherical structures (Figure 4a–b). In contrast, when the outlet temperature was below the boiling point, the pressure difference led to particle shrinkage (Figure 4c–d) [3].
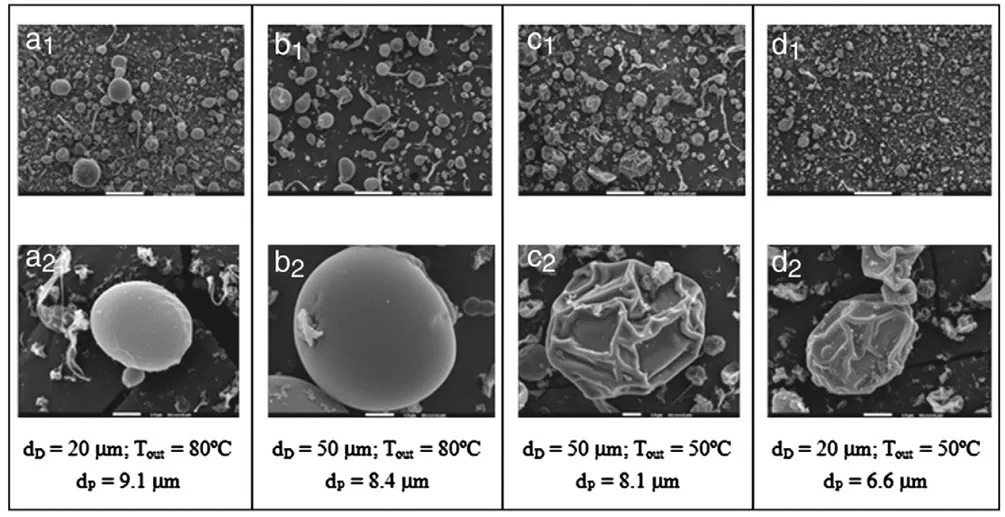
Figure 4. SEM Images of HPMCP Spray-Dried Particles Under Different Process Conditions
Optimizing spray drying is a systematic endeavor that demands a holistic understanding of the interplay between material properties, equipment design, and product quality. Deeper insights into the mechanisms governing four key parameters—inlet temperature, atomization pressure, feeding speed, and outlet temperature—can substantially enhance both the efficiency and reliability of process development.
Looking ahead, the broader application of Quality by Design (QbD) principles, combined with advances in intelligent control technologies, will enable more precise parameter control and greater product consistency. These developments will strengthen the technical foundation for formulation development and commercial manufacturing in the pharmaceutical industry.
[1] Crystal Pharmatech(2023) Amorphous Solid Dispersion Formulation Development and Production Application Cases(Part 2)—Spray Dryinghttps://mp.weixin.qq.com/s__biz=MzA3MzI1MTgxMA==&mid=2652826594&idx=1&sn=4ce6941f07fe509cbb7f9b8c08612ea5&scene=21& poctoken=HAFClGij1eBUuAifBMmvHb5cmAADZ2XoCMlSWej7
[2] Baldinger, A., Clerdent, L., Rantanen, J., Yang, M., & Grohganz, H. (2011). Quality by design approach in the optimization of the spray-drying process. Pharmaceutical Development and Technology, 17(4), 389–397.
https://doi.org/10.3109/10837450.2010.550623
[3] João Vicente, João Pinto, José Menezes, Filipe Gaspar. (2013). Fundamental analysis of particle formation in spray drying. Powder Technology, 247, 1-7. https://doi.org/10.1016/j.powtec.2013.06.038.
[4] Shah, N., Sandhu, H., Choi, D. S., Chokshi, H., & Malik, A. W. (2014) Amorphous Solid Dispersions: Theory and Practice. Springer New York.
https://doi.org/10.1007/978-1-4939-1598-9.
Subscribe to be the first to get the updates!