News
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
04 Dec 2025
 Crystal Formulation Services(CFS)Opens New Formulation Development Center and Analytical R&D Lab in Suzhou, China
We have both formulation laboratory and pilot GMP-like workshop equipped with a number of international top equipments for oral solid dosage development. The formulation laboratory is mainly used for ...
Crystal Formulation Services(CFS)Opens New Formulation Development Center and Analytical R&D Lab in Suzhou, China
We have both formulation laboratory and pilot GMP-like workshop equipped with a number of international top equipments for oral solid dosage development. The formulation laboratory is mainly used for ...
 Immuno-Oncology Summit 2024 - Meet Crystal Bio at Booth 15
Analytical Methods to Assess Critical Quality Attribute in mRNA-LNP ModalityJoin Crystal Bio's Co-founder, CTO, and Head of USA BD Ye Gu, Ph.D., as she presents at the Immuno-Oncology Summit 2024...
Immuno-Oncology Summit 2024 - Meet Crystal Bio at Booth 15
Analytical Methods to Assess Critical Quality Attribute in mRNA-LNP ModalityJoin Crystal Bio's Co-founder, CTO, and Head of USA BD Ye Gu, Ph.D., as she presents at the Immuno-Oncology Summit 2024...
 Fun Sharing: The Story of Ice Crystals and Frozen Foods (Part 2)
Ice crystals: Some times I really can't help myself, so let's hear it from me.The influence on food freezing: ice crystal behavior during fast/slow freezing and thawing of foodFor the growth o...
Fun Sharing: The Story of Ice Crystals and Frozen Foods (Part 2)
Ice crystals: Some times I really can't help myself, so let's hear it from me.The influence on food freezing: ice crystal behavior during fast/slow freezing and thawing of foodFor the growth o...
 The Bioprocessing Summit 2024 - Meet Crystal Bio at Booth T13
Join Crystal Bio's Co-founder, CTO, and Head of USA BD Ye Gu, Ph.D., as she presents at the Immuno-Oncology Summit 2024.Title: Analytical Methods to Assess Critical Quality Attribute in mRNA-LNP ...
The Bioprocessing Summit 2024 - Meet Crystal Bio at Booth T13
Join Crystal Bio's Co-founder, CTO, and Head of USA BD Ye Gu, Ph.D., as she presents at the Immuno-Oncology Summit 2024.Title: Analytical Methods to Assess Critical Quality Attribute in mRNA-LNP ...
 Candoo's Formulation Technology Platform Featured in the Drug Development & Delivery Journal
Crystal Pharmatech, the leading solid state research CRO, is proud to announce that the formulation platform of its subsidiary Candoo Pharmatech Company Inc. to address solubility and bioavailability ...
Candoo's Formulation Technology Platform Featured in the Drug Development & Delivery Journal
Crystal Pharmatech, the leading solid state research CRO, is proud to announce that the formulation platform of its subsidiary Candoo Pharmatech Company Inc. to address solubility and bioavailability ...
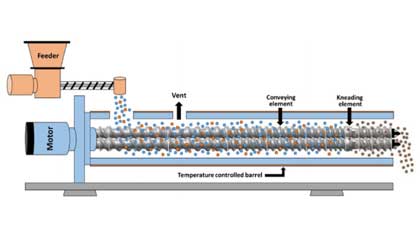 Case Study on Development and Production Applications of Amorphous Solid Dispersions - Hot Melt Extrusion
Hot melt extrusion (HME) is a common formulation technology for amorphous solid dispersions (ASD) with advantages such as continuity, high production efficiency, low energy consumption, and environmen...
Case Study on Development and Production Applications of Amorphous Solid Dispersions - Hot Melt Extrusion
Hot melt extrusion (HME) is a common formulation technology for amorphous solid dispersions (ASD) with advantages such as continuity, high production efficiency, low energy consumption, and environmen...
 Meet Crystal Pharmatech at 2024 Controlled & Modified Drug Release Summit
Presenting and discussing advancements in formulation science at the 2024 Controlled & Modified Drug Release Summit in Princeton, NJ.
We look forward to a CDMO case study presentation by Robert Wenslow, Ph.D. as well as two days of networking with fellow innovators as Dr. Wenslow is joined by Alex Chen, Ph.D. and Derik McCarthy.
Meet Crystal Pharmatech at 2024 Controlled & Modified Drug Release Summit
Presenting and discussing advancements in formulation science at the 2024 Controlled & Modified Drug Release Summit in Princeton, NJ.
We look forward to a CDMO case study presentation by Robert Wenslow, Ph.D. as well as two days of networking with fellow innovators as Dr. Wenslow is joined by Alex Chen, Ph.D. and Derik McCarthy.
 Crystal Formulation Services' GMP Manufacturing Facility Successfully Passes the Remote Audit by US Client, Marking a Key Milestone for Its International Expansion
Today, Crystal Pharmatech announced that the GMP manufacturing facility of their CDMO business unit – Crystal Formulation Services (CFS) has successfully passed a remote audit conducted by a US clien...
Crystal Formulation Services' GMP Manufacturing Facility Successfully Passes the Remote Audit by US Client, Marking a Key Milestone for Its International Expansion
Today, Crystal Pharmatech announced that the GMP manufacturing facility of their CDMO business unit – Crystal Formulation Services (CFS) has successfully passed a remote audit conducted by a US clien...
 Development and Production Application Case Study of Amorphous Solid Dispersions: Spray Drying
In the previous article, we introduced the application of hot-melt extrusion technology in the development of amorphous solid dispersions in pharmaceutical formulation. Spray drying (SD) is another co...
Development and Production Application Case Study of Amorphous Solid Dispersions: Spray Drying
In the previous article, we introduced the application of hot-melt extrusion technology in the development of amorphous solid dispersions in pharmaceutical formulation. Spray drying (SD) is another co...
Subscribe to be the first to get the updates!