News
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
04 Dec 2025
 Meet Crystal Pharmatech at Sorption Symposium North America 2024
Solid State Chemistry: Do You Like Solving Puzzles?Understanding the chemical composition and physical properties of crystalline API can be accomplished by using a variety of instruments and methods. ...
Meet Crystal Pharmatech at Sorption Symposium North America 2024
Solid State Chemistry: Do You Like Solving Puzzles?Understanding the chemical composition and physical properties of crystalline API can be accomplished by using a variety of instruments and methods. ...
 Crystal Pharmatech Receives CNAS Reaccreditation, Scope Expansion, and Address Approval
Suzhou, China – August 11, 2025 – Crystal Pharmatech Co., Ltd. announced that its China Suzhou CRO business unit has successfully completed the re-assessment, scope expansion, and laboratory address...
Crystal Pharmatech Receives CNAS Reaccreditation, Scope Expansion, and Address Approval
Suzhou, China – August 11, 2025 – Crystal Pharmatech Co., Ltd. announced that its China Suzhou CRO business unit has successfully completed the re-assessment, scope expansion, and laboratory address...
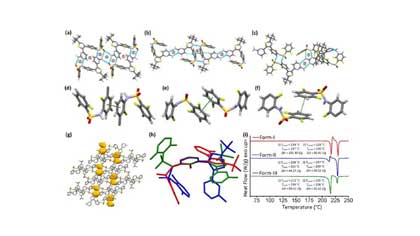 Polymorphs, Solvatomorphs and Hydrate of Dabrafenib
The solid-form screening and selection of active pharmaceutical ingredients (API) are crucial steps in the process of process development.
Polymorphs, Solvatomorphs and Hydrate of Dabrafenib
The solid-form screening and selection of active pharmaceutical ingredients (API) are crucial steps in the process of process development.
 CPHI North America - Meet Crystal Pharmatech at Booth 1441
Catch up with Crystal Pharmatech Subject Matter Expert Derik McCarthy in Philadelphia from May 7-9 at CPHI North America.We will be at Booth 1441 with LAVIANA PHARMA CO, LTD
CPHI North America - Meet Crystal Pharmatech at Booth 1441
Catch up with Crystal Pharmatech Subject Matter Expert Derik McCarthy in Philadelphia from May 7-9 at CPHI North America.We will be at Booth 1441 with LAVIANA PHARMA CO, LTD
 Assessment of CQA in mRNA-LNP Modality
“Figure 1. mRNA encapsulated with LNP typically composed of encapture nucleic acids such as mRNA, siRNA, etc. as shown in the blue curve line by lipid nanoparticle, LNP as ionized, pegylated, phosph...
Assessment of CQA in mRNA-LNP Modality
“Figure 1. mRNA encapsulated with LNP typically composed of encapture nucleic acids such as mRNA, siRNA, etc. as shown in the blue curve line by lipid nanoparticle, LNP as ionized, pegylated, phosph...
 Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
Suzhou, China - Recently, Crystal Pharmatech's CDMO Business Unit - Crystal Formulations Services (CFS) announced that it is honored to have received high recognition from their client, Brise Phar...
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
Suzhou, China - Recently, Crystal Pharmatech's CDMO Business Unit - Crystal Formulations Services (CFS) announced that it is honored to have received high recognition from their client, Brise Phar...
 Shining Glory: Unveiling the “True Eye” behind the Veil of Drug Crystal Forms
Are you still troubled by the characterization of solid state?How to determine whether the prepared crystal form is the same?Compounds have polymorphism. Is it necessary to conduct impurity crystal fo...
Shining Glory: Unveiling the “True Eye” behind the Veil of Drug Crystal Forms
Are you still troubled by the characterization of solid state?How to determine whether the prepared crystal form is the same?Compounds have polymorphism. Is it necessary to conduct impurity crystal fo...
 Meet Crystal Pharmatech at MIDD+ Boston 2024
Join Crystal Pharmatech Subject Matter Expert, Ridwan Islam, M. Pharm., Ph.D., at MIDD+ Boston, hosted by Simulations Plus, Inc. on May 6-7.Register for scientific presentations and hands-on learning ...
Meet Crystal Pharmatech at MIDD+ Boston 2024
Join Crystal Pharmatech Subject Matter Expert, Ridwan Islam, M. Pharm., Ph.D., at MIDD+ Boston, hosted by Simulations Plus, Inc. on May 6-7.Register for scientific presentations and hands-on learning ...
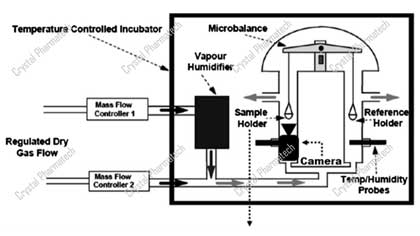 Applications of Dynamic Moisture Adsorption in Crystal Research
Content Overview1. Introduction2. Regulatory Requirements3. Application of Dynamic Vapor Sorption (DVS)3.1 Instrument Introduction3.2 Application 1 - Moisture Sorption Assessment3.3 Application 2 - Va...
Applications of Dynamic Moisture Adsorption in Crystal Research
Content Overview1. Introduction2. Regulatory Requirements3. Application of Dynamic Vapor Sorption (DVS)3.1 Instrument Introduction3.2 Application 1 - Moisture Sorption Assessment3.3 Application 2 - Va...
 Formulation Development: Fastest-to-FIH Without Sacrificing Quality
Crystal Pharmatech is happy to host a presentation & panel discussion at the #BiotechTuesday event on April 2 from 5 - 6 PM followed by a networking session at the La Fabrica Central, Cambridge, MA.
Formulation Development: Fastest-to-FIH Without Sacrificing Quality
Crystal Pharmatech is happy to host a presentation & panel discussion at the #BiotechTuesday event on April 2 from 5 - 6 PM followed by a networking session at the La Fabrica Central, Cambridge, MA. Parameter Sensitivity with GastroPlus
How can physiologically-based pharmacokinetic (PBPK) modeling maximize ADME performance and identify a path forward for GLP Tox and FIH formulations?GastroPlus incorporates in-vivo and in-vitro data t...
Parameter Sensitivity with GastroPlus
How can physiologically-based pharmacokinetic (PBPK) modeling maximize ADME performance and identify a path forward for GLP Tox and FIH formulations?GastroPlus incorporates in-vivo and in-vitro data t...
Subscribe to be the first to get the updates!