Content Overview
1. Introduction
2. Regulatory Requirements
3. Application of Dynamic Vapor Sorption (DVS)
3.1 Instrument Introduction
3.2 Application 1 - Moisture Sorption Assessment
3.3 Application 2 - Vapor-Induced Phase Transition Studies
3.4 Application 3 - Determining Amorphous Content
4. Considerations
5. Conclusion
6. References
Introduction
The Dynamic Vapor Sorption (DVS) analyzer is used to investigate the impact of moisture on a sample by precisely measuring the changes in weight before and after the sample is exposed to controlled temperature and humidity conditions. DVS offers advantages such as rapid detection, high sensitivity, minimal sample usage, and automation, making it suitable for studying moisture adsorption/desorption properties.
Moisture in the air is a constant and significant factor throughout the entire drug research process, spanning production, storage, and usage. Therefore, studying moisture adsorption/desorption phenomena is crucial for a better understanding of a sample's physicochemical properties, formulation process development, and determining suitable storage conditions.
Regulatory Requirements
Various pharmaceutical regulatory agencies, both domestic and international, have specific guidelines related to the study of crystalline forms, including requirements for investigating the hygroscopicity of drugs.
"Guidelines for the Testing of Hygroscopicity of Drugs" in the Chinese Pharmacopoeia (CP 9103) and FDA's Abbreviated New Drug Applications (ANDAs) contain explicit requirements for evaluating the hygroscopicity of pharmaceuticals.
CP 9103: The hygroscopicity of a drug refers to its ability or degree of absorbing moisture under certain temperature and humidity conditions. For solid active pharmaceutical ingredients that comply with drug quality standards, the test results can serve as a reference for selecting suitable drug packaging and storage conditions.
FDA ANDAs: Pharmaceutical Solid Polymorphism Chemistry, Manufacturing, and Controls Information: Drug substance polymorphic forms can also exhibit different physical and mechanical properties, including hygroscopicity, particle shape, density, flowability, and compactibility, which in turn may affect processing of the drug substance and/or manufacturing of the drug product.
Application of Dynamic Vapor Sorption (DVS)
Instrument Introduction
The Dynamic Vapor Sorption analyzer combines microbalances, gas flow, and vapor measurement techniques to study moisture in pharmaceuticals. Its core component is an extremely sensitive and recordable microbalance, capable of measuring changes in sample mass as low as one part per ten million.
In the DVS method, the test sample is placed on the microbalance. A gas (nitrogen) with a known concentration of vapor (water vapor) flows through the sample at a set flow rate and temperature. The change in sample mass on the microbalance is then recorded, revealing the moisture adsorption/desorption behavior of the sample.
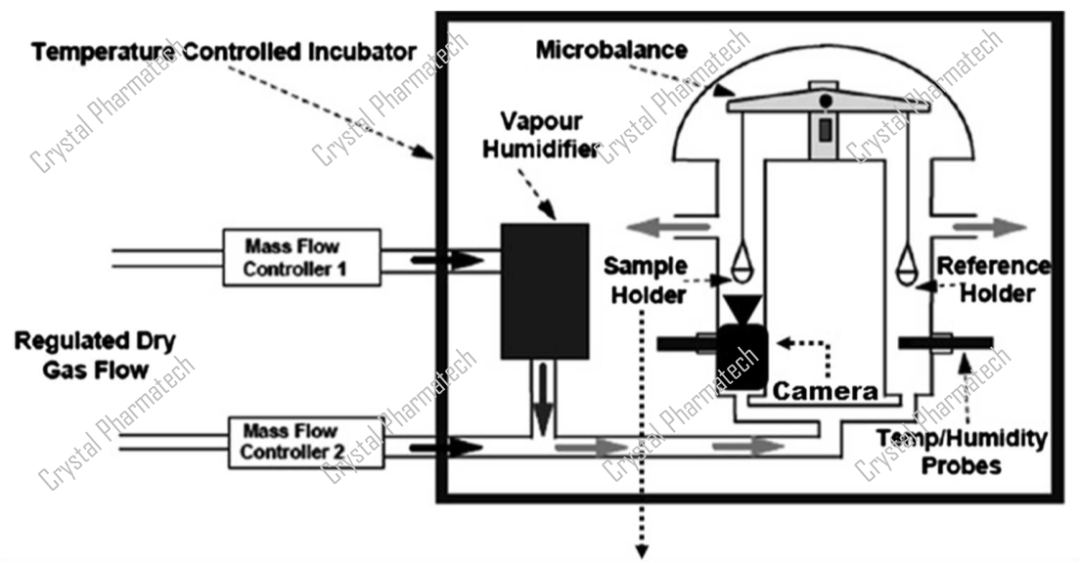 Schematic diagram of DVS Advantage apparatus
Schematic diagram of DVS Advantage apparatus
Application 1 - Moisture Sorption Assessment
Moisture sorption data obtained through Dynamic Vapor Sorption (DVS) analysis can provide valuable support for selecting preferred crystalline forms, determining sample storage conditions, and studying the kinetic stability humidity range of anhydrous and hydrated forms. Particularly for salt compounds such as hydrochlorides and sodium salts, the introduction of ligands can make it easier for the resulting free forms to form hydrogen bonds with water molecules, leading to significant weight gain under high humidity conditions. This poses challenges to crystalline stability and raw material storage. DVS data can help identify these potential issues early in the development process.
Using DVS testing, Crystalline Cloud can quickly assess the hygroscopicity of a crystalline form in the early stages of development. Combined with characterization techniques such as X-ray powder diffraction (XRPD), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC), it is possible to understand the stability range of crystalline forms, discover new polymorphs, and study the crystalline water of hydrates.
Application 1 - Moisture Sorption Assessment
According to the United States Pharmacopeia (USP), moisture is not considered an impurity. However, it is essential to closely monitor and control the presence of moisture in drugs. The moisture content can affect crystallinity, as well as drug permeability, density, and melting point. Particularly for amorphous forms, moisture can significantly alter their glass transition temperature and even induce recrystallization. Additionally, for some compounds, moisture can promote hydrolysis and induce drug degradation.
For the assessment of moisture sorption, the traditional experimental method involves the use of a desiccator containing a saturated salt solution and an analytical balance. This static determination method requires a large sample quantity, long testing times, and is sensitive to environmental conditions. Using DVS for testing offers several advantages, including convenience, the ability to place the sample on a microbalance, setting a specific water vapor adsorption/desorption program, requiring a smaller sample quantity, simplicity of operation, and environmental stability. DVS has become a commonly used method for assessing drug hygroscopicity. Under the assumption of an unchanged crystalline form, the hygroscopicity is defined in the pharmacopeias as follows:
Slightly hygroscopic: Less than 2% but not less than 0.2% weight gain.
Hygroscopic: Less than 15% but not less than 2% weight gain.
Very hygroscopic: Not less than 15% weight gain.
Deliquescent: Absorbs sufficient moisture to form a liquid.
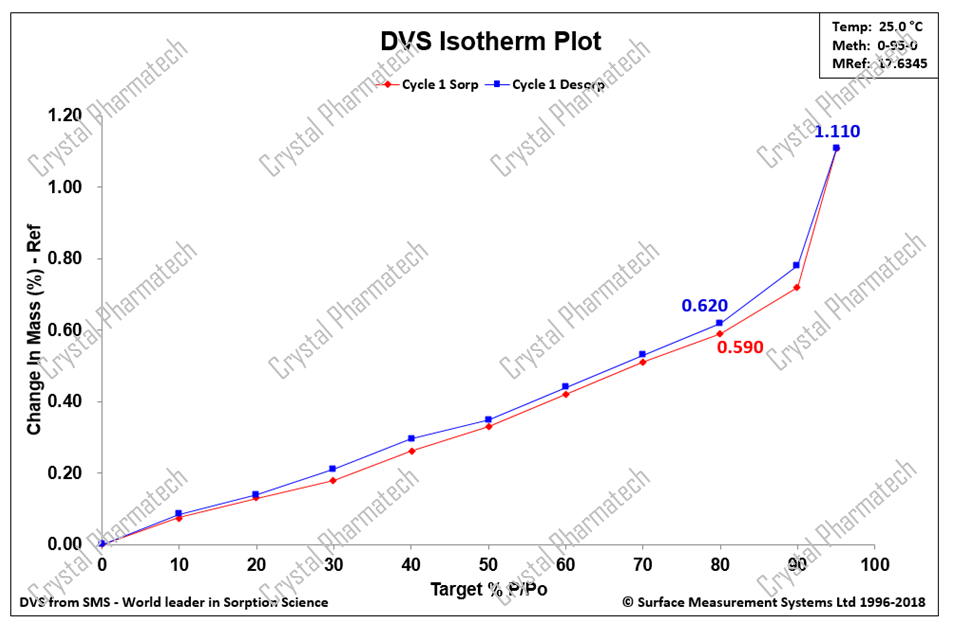
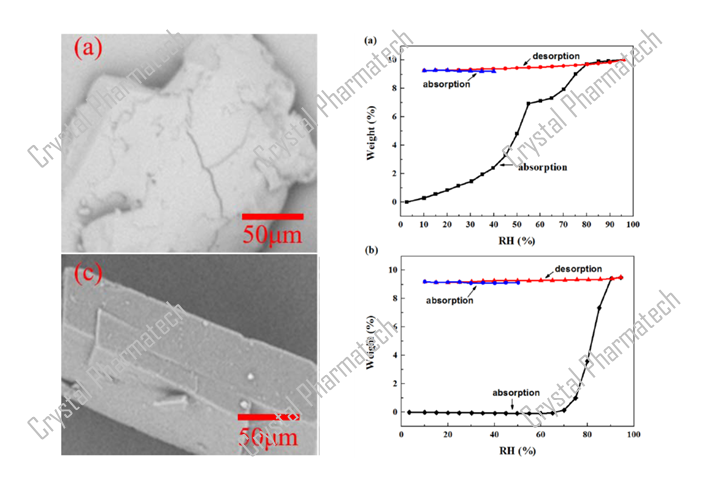
Taking Anhydrous Crystal Form I as an example, after adsorption/desorption cycles (0% RH-95% RH-0% RH), the weight increased by 0.59% at a relative humidity of 80%. According to the guidance for drug hygroscopicity tests, Crystal Form I is considered slightly hygroscopic.
Taking the example of Crystal Form A as a hydrate, there is no significant change in mass within the range of 10-90% relative humidity (RH). This indicates that Crystal Form A is kinetically stable within this range. Moreover, with the help of techniques such as thermogravimetric analysis (TGA) and nuclear magnetic resonance (NMR), it is possible to estimate the number of water molecules present in Crystal Form A.
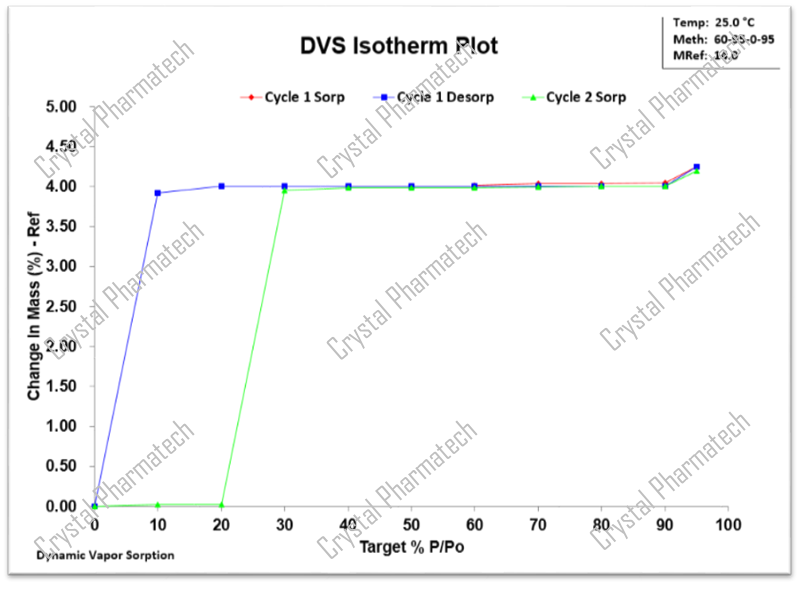
Based on the characterization results from DVS, the key thermodynamic water activities between the hydrate Crystal Form A and the anhydrous form obtained after dehydration are in the range of 10-20%.
Application 2 - Vapor-Induced Phase Transformation Studies
Determining the humidity stability range of hydrates is an essential step in hydrate development, as the moisture content directly affects the choice of raw material/drug product processes and storage conditions. During the early stages of polymorph screening, various crystalline forms are usually determined based on slurry competition to establish their transformation relationships. For crystalline forms that are not suitable for slurry competition, such as those with high solubility or prone to form solvates, the thermodynamic stability humidity range of hydrates can be evaluated using DVS.
Furthermore, by combining DVS characterization data, it is possible to gain initial insights into whether a hydrate transforms into an anhydrate or other hydrates. Conversely, for an anhydrate, it can provide preliminary information on whether it transforms into a hydrate.
Case 1: Investigation of Polymorphic Transformation Relationships
Based on the DVS data for hydrate Crystal Form A, during the desorption of moisture, Crystal Form A dehydrates at 10% RH, resulting in the anhydrous Crystal Form B. As the humidity increases to 30% RH, Crystal Form B absorbs moisture and transforms into hydrate Crystal Form C. Continuing to increase the humidity from 60% RH to 90% RH, Crystal Form C absorbs moisture and transforms back into Crystal Form A. Based on these transformation intervals, it can be concluded that under low humidity conditions (less than 10% RH), it transforms into Crystal Form B, and under humidity conditions between 20% RH and 60% RH, it transforms into Crystal Form C.
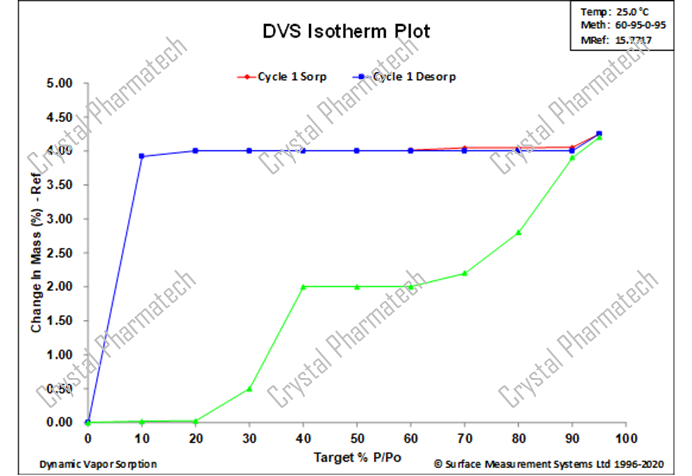
Case 2: Combining DVS with Hygroscopic XRPD
For crystalline forms that are sensitive to humidity, transferring the sample to environmental conditions for XRPD testing may lead to crystalline transformations, making it challenging to capture potential metastable forms. In such cases, hygroscopic XRPD can be used to collect XRPD data under different humidity conditions in situ, enabling the detection of potential crystalline forms.
Combining DVS data (as shown in figure b), when the humidity increases from 50% to 60% or from 90% to 95%, there is a significant increase in sample weight due to moisture uptake, indicating the possibility of a polymorphic transformation. In this project, in conjunction with hygroscopic XRPD characterization, XRPD data was collected under various humidity conditions to identify the new crystalline form.
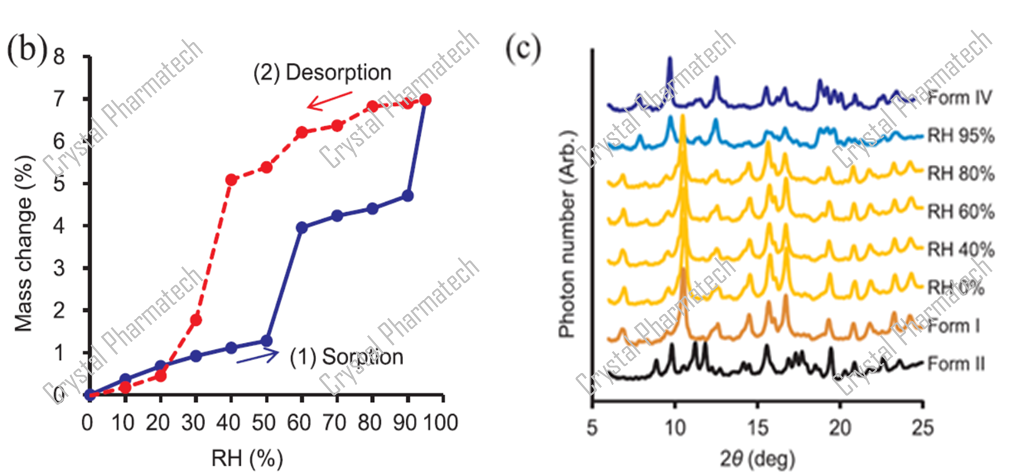
Case 3: Amorphous to Crystalline Transformation
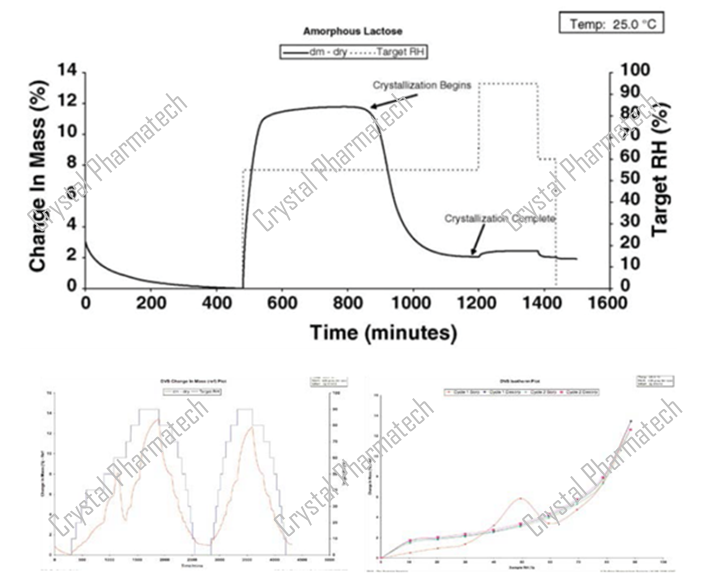
Amorphous crystallization is one method for obtaining new crystalline forms. In addition to conducting screening experiments with an amorphous starting point, the possibility of crystallization can also be assessed using DVS. Taking amorphous lactose as an example, during the DVS testing process, there is a decrease in mass as humidity increases. This phenomenon occurs because the amorphous form crystallizes under the influence of moisture.
Application 3 - Detection of Amorphous Content
The presence of a certain amount of amorphous content in a drug can be necessary to improve its solubility. However, it can also result from various mechanical forces during manufacturing processes such as grinding or granulation. The amorphous content can affect various aspects of the drug, including its efficacy, processing, and storage, so it's essential to accurately determine the amorphous content. Common methods like X-ray Powder Diffraction (XRPD) have a limit of detection (LOD) of around 10%, while Differential Scanning Calorimetry (DSC) has an LOD of approximately 5%. In contrast, the DVS method can achieve an LOD as low as 0.05%, making it suitable for detecting low levels of amorphous content.
The DVS method for measuring amorphous content includes:
Measuring the mass change of the amorphous sample under specific and suitable conditions and comparing it with a calibration curve of known amorphous content samples.
Comparing the curves before and after vapor-induced crystallization.
A method reported in the literature by Mackin et al. involves inducing crystallization of the amorphous phase using a solvent and measuring the solvent adsorption before and after crystallization to determine the amorphous content.
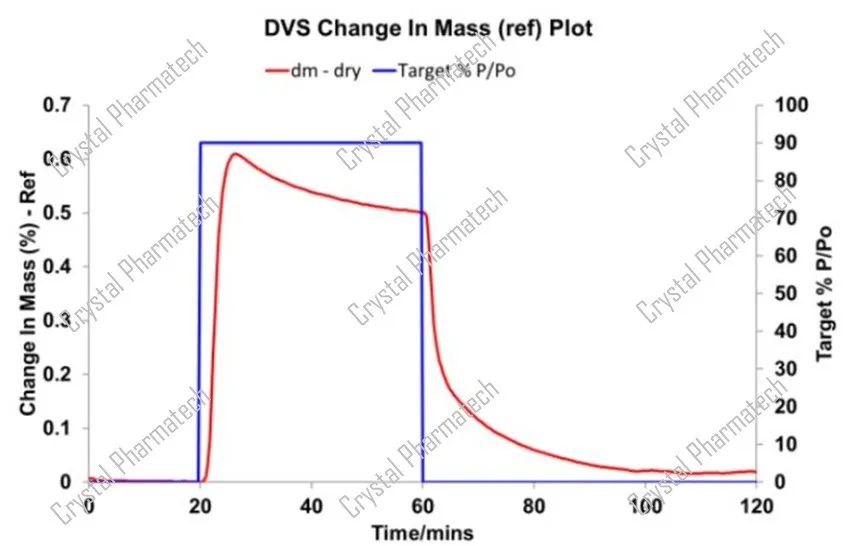
Taking fluticasone propionate as an example, its crystallization solvent is ethanol. The graph below shows that when the compound is exposed to 90% P/Po ethanol, there is a noticeable mass decrease related to crystallization.
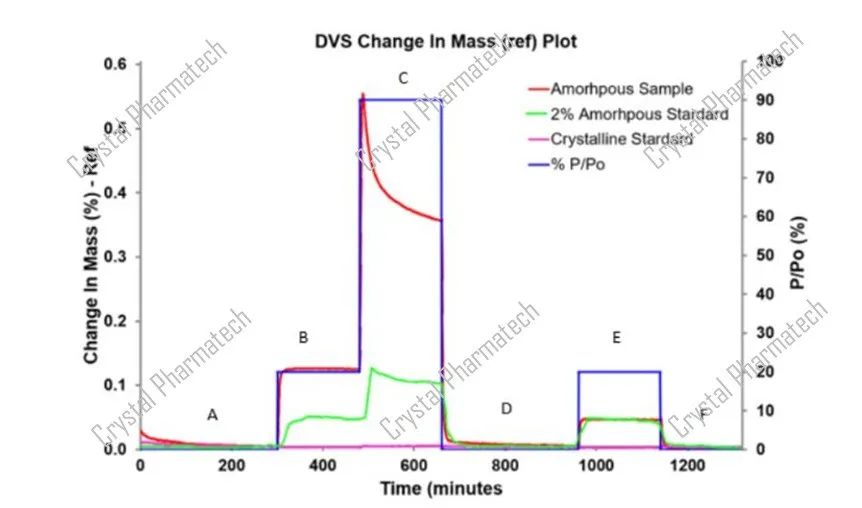
In the experiment, standard curves were prepared for fluticasone propionate at concentrations of 2%, 5%, 10%, and fully crystalline. The graph below illustrates the DVS results for the test sample, the fully crystalline standard, and the 2% amorphous standard. Both the test sample and the 2% amorphous standard show a significant decrease in mass indicative of crystallization at 90% P/Po, while the fully crystalline standard exhibits minimal change in mass. Using the DVS analysis software, the amorphous content in the test sample can be determined.
Considerations
Drawing from CrystalYun's solid-state research experience over the past 12 years, the following summarizes key considerations and insights for DVS (Dynamic Vapor Sorption) testing:
Selection of Adsorption/Desorption Program:
When conducting crystalline evaluations, the choice of adsorption/desorption program should align with the properties of the test sample. For anhydrous forms, it is advisable to initiate the program at 0% RH, while for hydrates, commencing with ambient humidity or a known stable crystalline form's humidity is recommended.
Residual Solvent Factors:
Although DVS characterization has relatively low material requirements, it is not advisable to use samples with significant residual solvents. If residual solvents cannot be removed during the pre-equilibration stage, the controlled humidity environment using dry/wet nitrogen gas during DVS testing may cause solvent removal. This can lead to mass changes being measured, which could result in data differing from the actual situation.
Sample Characteristics:
Even for the same crystalline form, sample factors such as surface area, particle size, and crystalline habits can cause variations in DVS profiles. Researchers have noted that two samples of the same anhydrous form prepared using different methods (one with a rough surface and the other with a smooth surface) both converted to the same hydrate after moisture uptake. However, the DVS adsorption process exhibited differences, reflecting variations in the kinetics of water uptake. When using DVS to determine storage humidity conditions based on the results, it's essential to consider the influence of sample crystalline habits.
These considerations are crucial for accurate and meaningful DVS testing, especially in the context of pharmaceutical solid-state research and development.
a: Rough Surface, b: Smooth Surface - Differences in DVS Curves upon Hydration to Form the Hydrate
Synchronized Data Collection
In the early stages of pharmaceutical research, when the study of crystalline stability is not comprehensive, it is advisable to collect XRPD data before and after DVS characterization. This allows for the integration of DVS data with XRPD, facilitating the analysis of crystalline stability regions and other relevant information.
Conclusion
Pharmaceutical polymorphism research is integral to various stages of drug substance and formulation development. DVS serves as an indispensable characterization technique in polymorphism research, and CrystalYun is equipped with multiple DVS instruments to provide partners with rapid and precise DVS characterization, supporting the development of polymorphic drugs.
References
Arnold Duralliua, Paul Matejtschukb, Daryl R. Williamsa, European Journal of Pharmaceutics and Biopharmaceutics Humidity induced collapse in freeze dried cakes: A direct visualization study using DVS
Saleki-Gerhardt, A., Ahlneck, C., and Zografi, G., 1994. Int. J. Pharm., 101, 237-247
Buckton, G. and Darcy, P., 1995. Int. J. Pharm., 123, 265-271
Sheokand, Modi, and Bansal, JOURNAL OF PHARMACEUTICAL SCIENCES, Dynamic Vapor Sorption as a Tool for Characterization and Quantification of Amorphous Content in Predominantly Crystalline Materials
Subscribe to be the first to get the updates!