
Original link
https://pubs.acs.org/doi/epdf/10.1021/acs.cgd.2c01289
The solid-form screening and selection of active pharmaceutical ingredients (API) are crucial steps in the process of process development. The unique physical and chemical properties of crystal forms, such as color, melting point, solubility, stability, bulk density, flowability, and bioavailability, play important roles in drug discovery and development. Therefore, it is important to screen suitable solid forms of active pharmaceutical ingredients by using various solvent crystallization, optimizing crystallization temperature, pressure, and other conditions, and utilizing new technologies. The question then arises: how much time and effort is required to obtain all the polymorphs of a molecule and determine the thermodynamically most stable polymorph? In this study, the authors conducted polymorph screening and research on dabrafenib (DBF), a model compound (a BRAF inhibitor class cancer drug developed by GlaxoSmithKline (GSK) for the treatment of metastatic melanoma in the form of a mesylate salt). According to McCrone, the number of polymorphs of a compound is proportional to the time and effort spent studying that compound. The authors conducted extensive polymorph screening experiments using various solvents and conditions, resulting in three anhydrous forms (Form I, II, III), one monohydrate, and nine solvates.
From the perspective of crystal engineering, the authors analyzed the molecular conformation and crystal packing to understand the phenomenon of polymorphism and assist in predicting the possibility of further discovering polymorphs. The hydrogen bonding in DBF polymorphs can be observed in Figure 1, where π···π stacking between terminal aryl rings is present in all three anhydrous forms of DBF, but the difference lies in the orientation of the aryl rings relative to the molecular backbone.
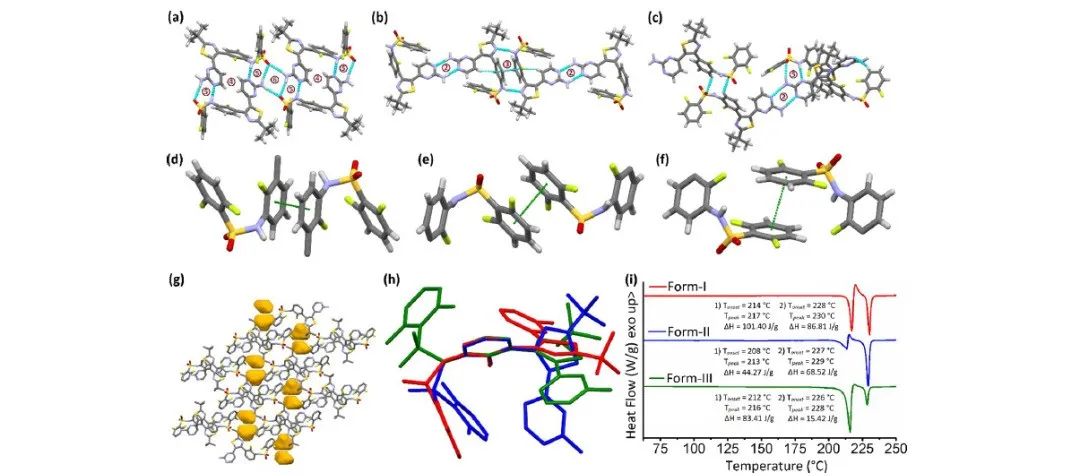 Figure 1. Selected interactions in DBF polymorphs (a) form I, (b) form II, (c) and form III, (d) π···π stacking between central aryl rings in form II,I exo π···π stacking of terminal aryl rings in form I and II, (f) endo π···π stacking of terminal aryl rings in form III, (g) voids in the crystal packing of form III, (h) molecular structure overlay of form I (red), II (blue), and III (green) on central aryl ring, and (i) stack plot of DSC thermogram ofform I (red), II (blue), and III (green).
Figure 1. Selected interactions in DBF polymorphs (a) form I, (b) form II, (c) and form III, (d) π···π stacking between central aryl rings in form II,I exo π···π stacking of terminal aryl rings in form I and II, (f) endo π···π stacking of terminal aryl rings in form III, (g) voids in the crystal packing of form III, (h) molecular structure overlay of form I (red), II (blue), and III (green) on central aryl ring, and (i) stack plot of DSC thermogram ofform I (red), II (blue), and III (green).
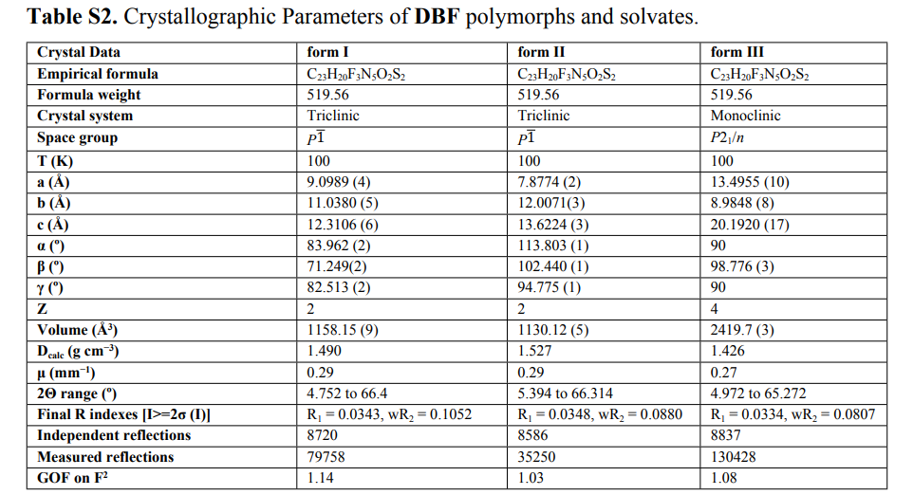
In the crystal structures of the three different polymorphs, the stacking and interactions of DBF (Dibenzofuran) molecules vary. According to the single-crystal data calculations (Table S2), polymorph II has the highest density, while polymorph III has the lowest density. Typically, in flexible-molecule polymorphs, as the crystal density decreases, the structure tends to adopt more open conformations. According to the Burger-Ramberger rule, the polymorph with the highest crystal density is thermodynamically more stable at absolute zero. However, in the differential scanning calorimetry (DSC) analysis, all three polymorphs (I, II, and III) exhibit phase transitions upon heating. Therefore, it is not straightforward to determine their melting points or establish clear thermodynamic relationships among these three polymorphs based solely on DSC observations.
The author then employed two computational models to calculate the thermodynamic relationships among polymorphs I, II, and III. In contrast to the conclusion derived from the Burger-Ramberger rule, both periodic density functional theory (DFT) lattice energy calculations and dispersion-corrected B86bPBE-XDM function predicted that polymorph III is the most stable among them.
However, when the degree of π-conjugation of conformations varies, the inherent delocalization error of generalized gradient approximation (GGA) density functionals like B86bPBE cannot accurately predict conformational energies. To overcome this issue of conformational energy, the author applied a conformational energy correction based on domain-based local pair natural orbital coupled-cluster theory (DLPNO-CCSD(T)), as shown in Equation 1.
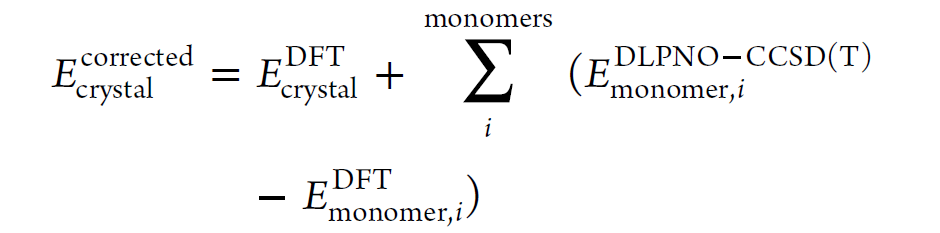
By calculating the crystal energy for each monomer in the unit cell and the gas-phase monomer energy, these terms were summed together, and then the relative energy of each molecule with respect to the most stable polymorph (polymorph II) was evaluated as shown in Equation 1. Since each crystal here contains only one molecule in the asymmetric unit (Z′=1), the gas-phase calculation needs to be performed only once, and the result can be multiplied by the number of symmetrically equivalent molecules.The correction for the 0K internal (electronic) energy of the crystal is equivalent to simulating the intramolecular conformational energy using DLPNO-CCSD(T), while intermolecular interactions are described using DFT. DLPNO's approximation to CCSD(T) typically reproduces traditional CCSD(T) quite well, albeit at a much lower computational cost, making it feasible for large molecules like DBF. DLPNO-CCSD(T) does not suffer from delocalization errors like DFT models, so the conformational energies it predicts should be more reliable.
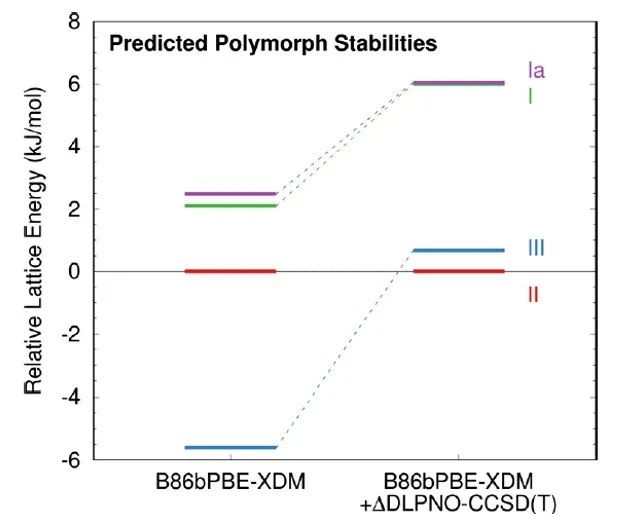
Figure 3. Calculated relative lattice energies of forms Ia, I, II, and III using B86bPBE-XDM directly and after applying the DLPNOCCSD(T) intramolecular conformational energy correction.
After conformational energy corrections were applied to the three DBF polymorphs, the predicted stability order is as follows: polymorph II > polymorph III ≫ polymorph I > polymorph Ia (Figure 3). Despite an energy difference of 0.7 kJ/mol in favor of polymorph II over polymorph III from an energy calculation perspective, limitations in the precision of the computational model and the focus on lattice energy rather than finite-temperature enthalpy or free energy hinder the accurate ranking of these two polymorphs in terms of energy. It should be noted that the similarly predicted energies for these two polymorphs are consistent with experimental observations (co-crystallization of the two polymorphs).
In summary,
To meet the needs of drug development and regulation, it is crucial to determine the crystal form of APIs and their stability relationships through systematic polymorph screening. DBF crystallizes into three anhydrous polymorphs (I, II, and III) from various solvents. First-principles calculations using B86bPBE-XDM + ΔDLPNOCCSD(T) indicate that the energies of polymorphs II and III are very close (with polymorph II slightly lower than polymorph III) and significantly lower than that of polymorph I. Further verification of the most stable polymorph would require experimental techniques such as slurry competition and solubility determination.
The Crystal Pharm team at CrystalCloud has years of experience in polymorph screening and polymorph transformation studies. We look forward to more collaboration and knowledge sharing with peers in the field of pharmaceutical solid-state research. We have also shared related technical articles on polymorph screening and transformation (Crystallographic Studies: Screening and Selection (Part One)and Crystallographic Studies: Screening and Selection (Part Two)), and we welcome everyone to follow and learn more.
Furthermore, in terms of predicting polymorph stability, there is an expectation for a deeper understanding of both force fields and quantum mechanics, leading to the development of more accurate and efficient computational models. With the rapid advancement of computational capabilities, it is hoped that complex system calculations, which are currently challenging, can be addressed. We have also shared related technical articles on polymorph prediction (Crystal Structure Prediction (What/Why/How) and Case Studies | Equipping Ourselves for Success: A Discussion on Crystal Structure Prediction Algorithms), and we welcome everyone to learn and progress together
Subscribe to be the first to get the updates!