News
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
04 Dec 2025
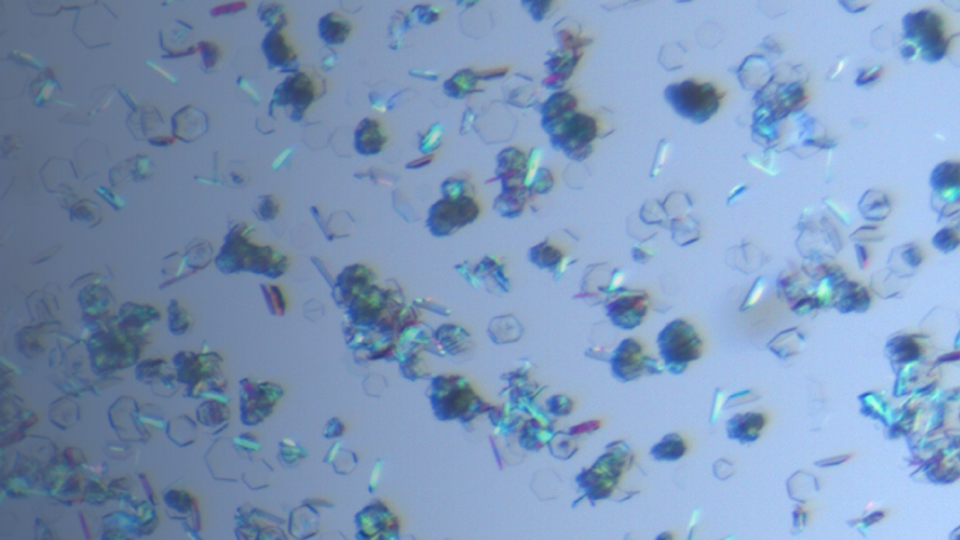 Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
 Bioavailability Enhancement for Insoluble Compounds & PROTAC & Oral Peptides
With the ever-increasing demand for the absorbability of small-molecule oral innovative drugs, more and more new molecules have hydrophobic properties, resulting in very low solubility. According to the latest statistics, about 70% of the small-molecule drugs under research are insoluble substances of BCS II or IV.
Bioavailability Enhancement for Insoluble Compounds & PROTAC & Oral Peptides
With the ever-increasing demand for the absorbability of small-molecule oral innovative drugs, more and more new molecules have hydrophobic properties, resulting in very low solubility. According to the latest statistics, about 70% of the small-molecule drugs under research are insoluble substances of BCS II or IV.
 Events
Events
 Case Study 3: Atorvastatin - Crystalline Form Change In Late Development
Atorvastatin (CI-981) is an HMG CoA reductase inhibitor marketed as Lipitor® (Fig. 8). As a BCS II drug, it has exhibited poor solubility and high permeability (Wu and Benet 2005). The compound was o...
Case Study 3: Atorvastatin - Crystalline Form Change In Late Development
Atorvastatin (CI-981) is an HMG CoA reductase inhibitor marketed as Lipitor® (Fig. 8). As a BCS II drug, it has exhibited poor solubility and high permeability (Wu and Benet 2005). The compound was o...
 Dr. LianFeng Huang Joins Crystal Pharmatech as Chief Scientist
Crystal Pharmatech is pleased to announce the appointment of Dr. LianFeng Huang as Chief Scientist. Dr. Huang is an accomplished R&D leader with over 30 years of experien...
Dr. LianFeng Huang Joins Crystal Pharmatech as Chief Scientist
Crystal Pharmatech is pleased to announce the appointment of Dr. LianFeng Huang as Chief Scientist. Dr. Huang is an accomplished R&D leader with over 30 years of experien...
 Concerned about mRNA -LNP Integrity during International Shipping?
Several batches of mRNA-LNP samples, shipped by CATUG (CDMO in China), were subjected to Quality Control-style testing performed at the receiving site (Crystal Bio, New Jersey, USA) to address the con...
Concerned about mRNA -LNP Integrity during International Shipping?
Several batches of mRNA-LNP samples, shipped by CATUG (CDMO in China), were subjected to Quality Control-style testing performed at the receiving site (Crystal Bio, New Jersey, USA) to address the con...
Subscribe to be the first to get the updates!