Atorvastatin (CI-981) is an HMG CoA reductase inhibitor marketed as Lipitor® (Fig. 8). As a BCS II drug, it has exhibited poor solubility and high permeability (Wu and Benet 2005). The compound was originally discovered at Warner-Lambert in the 1980’s, and the amorphous form of the hemi calcium salt pure enantiomer was used for early clinical trials. Phase 1 studies were conducted by the Parke-Davis Clinical Research Unit (CRU) recruiting twenty-four (24) males from within the company (Lie 2009). Phase 2 clinical trials showed an improvement in performance when compared to data from four marketed drugs (Fig. 9). Priority review status was requested in 1994, but was denied because the drug had not met an unmet medical need. The company proceeded to fund a clinical study for familial hypercholesterolemia, where the compound showed efficacy, and they were granted priority review status which helped to shorten the development time. Atorvastatin calcium was approved by the Food and Drug Administration (FDA) in late 1996.
Fig. 8: Structure of atorvastatin
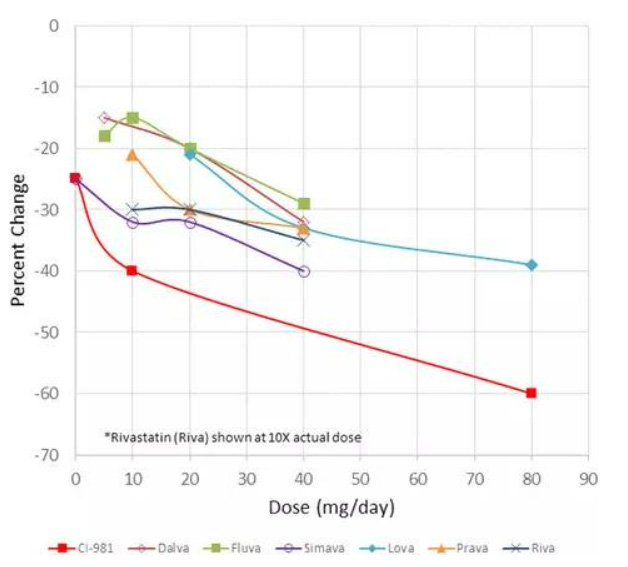
Fig. 9: Atorvastatin (CI-981) Phase II results compared with marketed products (adapted from Ref (Lie 2009))
The only known solid form for atorvastatin calcium in Phase 1 and 2 clinical trials was the amorphous form. It exhibited poor filtration and drying characteristics for large scale batches and required protection from heat, light, oxygen, and moisture (Briggs et al. 1999). During Phase 3 clinical trials, a crystalline form was produced at scale which was determined to be a trihydrate and referred to as Form I (Briggs et al.1999). This crystalline form possessed a number of advantages over the amorphous form including higher purity, improved chemical stability, tighter uniformity in particle size distribution, and better filtration and drying properties. While finding a new form at this stage of development would normally be undesirable, the improvements gained with the new crystalline form were substantial enough for researchers to change the solid form during late development. All aspects of the project needed to be repeated, such as the API manufacturing process development, formulation development, stability studies, analytical methods, and human bioequivalence testing. Tablets produced with amorphous and crystalline trihydrate atorvastatin calcium showed a difference in the rate of absorption, but equivalent extent of absorption in the bioequivalence test (Pfizer Citizen Petition. Docket no 2005P).
Other crystalline forms were patented along with Form I (designated Forms II and IV) (Briggs et al. 1999), and additional forms followed in subsequent patent applications (Byrn et al. 2003; Tesslor et al. 2003; Van Der Schaaf et al. 2009). The next challenge for the team was to develop a crystallization process that produced uniquely Form I with the desired characteristics they needed. One patented process reported that adding methyl-t butyl ether (MTBE) to the reaction mixture after forming the salt, followed by subsequent seeding, had produced the desired Form I (Tully 2003).
The FDA orange book has listed a number of patents for atorvastatin calcium, including the composition of matter patent (expired September 24, 2009), a salt patent including the calcium salt (expired Dec 28, 2010), and the crystalline Form I patent (expires July 8, 2016). By using a form other than Form I, generic products were technically allowed on the market in 2010. After numerous legal battles and an agreement between Pfizer and Ranbaxy, the generic version of Lipitor® was available in late 2011 (Lie 2009).
The atorvastatin story has covered a number of teaching points regarding solid forms. Polymorph screens were not routinely performed when atorvastatin was under development and it was common to find forms during scale-up, especially when conditions were changed. In the case of atorvastatin, a screen was performed after the crystalline form was found and a number of forms were produced, based on the patent literature (Briggs et al. 1999; Byrn et al. 2003; Tesslor et al. 2003; Van Der Schaaf et al. 2009). In present day cases, a solid form screen should be performed in early development to find a suitable form long before Phase 3 clinical trials. An earlier solid form screen would also have prevented the repeat of major studies late in development, as seen when atorvastatin Form I was found. Screening studies do not guarantee that all forms have been found, but they have significantly reduced the risk for most programs. The patents listed in the Orange Book and the strategy of using patents to maintain market share have also been recognized as an important lesson from this example since it has necessitated consideration of a patent strategy whenever new forms (polymorphs, hydrates, solvates, salts, cocrystals, amorphous solid dispersions, etc.) have been found during development.
While the initial discovery of a crystalline form during Phase 3 clinical trials would have normally been considered a “bad” scenario, the atorvastatin story has proven that after the extra work has been completed, a very “good” scenario and a successful product resulted.
Subscribe to be the first to get the updates!