News
Brise Pharma Presents“Best Partner”Trophy to Crystal Formulation Services, Recognizing Their Excellence in Drug Product CDMO Services
04 Dec 2025
 Industry Shift: FDA Goes Vegan?
Approaching Drug Development With FinesseIn 2012, drug development specialists started an initiative to create predictive Oral Bioavailability Tools (OrBiTo). This initiative recognized the need to mo...
Industry Shift: FDA Goes Vegan?
Approaching Drug Development With FinesseIn 2012, drug development specialists started an initiative to create predictive Oral Bioavailability Tools (OrBiTo). This initiative recognized the need to mo...
 Crystallization Development Services
Based on in-depth research on the properties of the crystal form itself and the transformation relationship between crystal forms, Crystal Pharmatech can offer a robust and scalable crystallization process that yields a reproducible high-quality solid form.
Crystallization Development Services
Based on in-depth research on the properties of the crystal form itself and the transformation relationship between crystal forms, Crystal Pharmatech can offer a robust and scalable crystallization process that yields a reproducible high-quality solid form.
 Development and Production Application Cases of Amorphous Solid Dispersion Formulations (II) - Spray Drying
In the preceding article, we discussed the application of hot-melt extrusion technology in developing amorphous solid dispersions (ASD) for pharmaceutical formulations. The process involves dispersing...
Development and Production Application Cases of Amorphous Solid Dispersion Formulations (II) - Spray Drying
In the preceding article, we discussed the application of hot-melt extrusion technology in developing amorphous solid dispersions (ASD) for pharmaceutical formulations. The process involves dispersing...
 Utilizing Compaction Simulation for Advancing Oral Dosage Formulation Design
Utilizing Compaction Simulation for Advancing Oral Dosage Formulation Design
 Discovery & Development US 2025
We're heading to San Diego October 2-3 and bringing a team ready to talk First-Time-Right CMC for small molecules—from solid form to formulation and IND.Date: October 2-3Location: Sheraton San Di...
Discovery & Development US 2025
We're heading to San Diego October 2-3 and bringing a team ready to talk First-Time-Right CMC for small molecules—from solid form to formulation and IND.Date: October 2-3Location: Sheraton San Di...
 About Us
Crystal Pharmatech, founded in 2010, is a global contract research organization with approximately 300 employees and four R&D centers in New Jersey (USA), San Francisco (USA), Toronto (Canada), and Suzhou (China). Collectively, these sites provide integrated support for pharmaceutical and biotechnology companies worldwide. We offer API solid-state research, crystallization, preformulation, formulation development and manufacturing, clinical supply, bioanalytical and biomarker testing, biologics CMC analytics, and clinical pharmacology.
About Us
Crystal Pharmatech, founded in 2010, is a global contract research organization with approximately 300 employees and four R&D centers in New Jersey (USA), San Francisco (USA), Toronto (Canada), and Suzhou (China). Collectively, these sites provide integrated support for pharmaceutical and biotechnology companies worldwide. We offer API solid-state research, crystallization, preformulation, formulation development and manufacturing, clinical supply, bioanalytical and biomarker testing, biologics CMC analytics, and clinical pharmacology.
 Pre-formulation Studies
Crystal Pharmatech can bridge the gap between the API and formulation through our solid-state service, which can be seamlessly progressed into pre-formulation research while de-risking your assets.
Pre-formulation Studies
Crystal Pharmatech can bridge the gap between the API and formulation through our solid-state service, which can be seamlessly progressed into pre-formulation research while de-risking your assets.
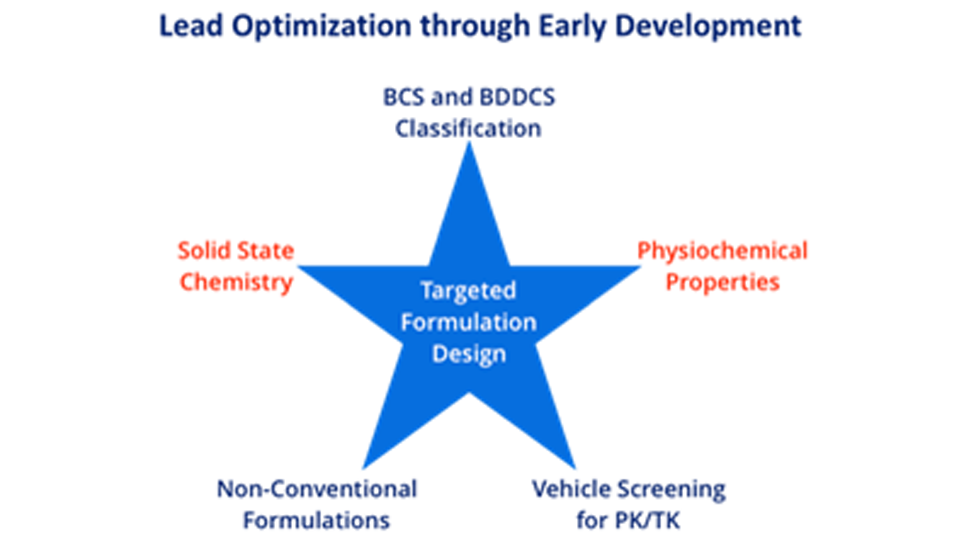 Formulations for PK/Efficacy/Tox Studies
We adopt integrated approach to find the right form and formulation through our SMART services, leading to the shortest possible development timelines with limited resource utilization.
Formulations for PK/Efficacy/Tox Studies
We adopt integrated approach to find the right form and formulation through our SMART services, leading to the shortest possible development timelines with limited resource utilization.
 Case Study 6: Oxybutynin- Crystalline Change From Salt to Free Base
Oxybutynin HCl (Fig. 14) has been recognized as a BDDCS I compound exhibiting high solubility and permeability (Benet et al. 2011). It has been used in a variety of marketed products for the treatment...
Case Study 6: Oxybutynin- Crystalline Change From Salt to Free Base
Oxybutynin HCl (Fig. 14) has been recognized as a BDDCS I compound exhibiting high solubility and permeability (Benet et al. 2011). It has been used in a variety of marketed products for the treatment...
 Crystal Pharmatech's CDMO Business Unit - Crystal Formulation Services Received China Drug Product Manufacturing License, Achieving the Important Milestone in Formulation Capability
Crystal Formulation Services, a subsidiary of Crystal Pharmatech, has achieved an exciting new milestone in its formulation capabilities. The company was recently granted the Drug Manufacturing ...
Crystal Pharmatech's CDMO Business Unit - Crystal Formulation Services Received China Drug Product Manufacturing License, Achieving the Important Milestone in Formulation Capability
Crystal Formulation Services, a subsidiary of Crystal Pharmatech, has achieved an exciting new milestone in its formulation capabilities. The company was recently granted the Drug Manufacturing ...
Subscribe to be the first to get the updates!