In the preceding article, we discussed the application of hot-melt extrusion technology in developing amorphous solid dispersions (ASD) for pharmaceutical formulations. The process involves dispersing a solution through a spray dryer, where it meets hot gas, quickly evaporates, and forms amorphous solids. Spray drying techniques are extensively employed in developing formulations for drugs with low solubility, largely due to their well-established scalability from small-scale experiments to large-scale commercial production, aligning with Good Manufacturing Practice (GMP) standards. Table 1 in the article provides examples of various drugs that have been commercially produced using spray drying technology to prepare amorphous solid dispersions.
Table 1:FDA-Approved ASD Products Made Through Spray Drying Methods
Product Name | Key Ingredients | BCS Class | FDA Approval Time | Manufacturer |
Incivek | Telaprevir | II | 2011 | Vertex |
Kalydeco | Lvacaftor | II | 2012 | Vertex |
Harvoni | Ledipasvir/Sofosbuvir | II/III | 2014 | Gilead Sciences |
Epclusa | Sofosbuvir/Velpatasvir | III/IV | 2016 | Gilead Sciences |
Orkambi | Lumacaftor/Lvacaftor | II/II | 2016 | Vertex |
Zepatier | Elbasvir/Grazoprevir | II/II | 2016 | Merck |
Erleada | Apalutamide | II | 2018 | Janssen |
Tukysa | Tucatinib | II | 2020 | Seagen |
Sotyktu | Deucravacitinib | II | 2022 | Bristol Myers Squibb |
Figure 1 shows the schematic layout of a spray drying system. The primary steps of the spray drying process includes:
1. Solution Preparation: Dissolving the Active Pharmaceutical Ingredient (API) and other excipients to form a homogeneous solution.
2. Spray Drying: Dispersing the solution into a fine spray through a nozzle, allowing the spray to contact with a hot drying gas, leading to rapid evaporation of the liquid and the formation of solid particles.
3. Cyclone Separation: Conveying the dried particles to a cyclone separator for separation from the gas.

Figure 2. Spray Drying Equipment Utilized at Crystal Pharmatech(Buchi B-290;GEA PSD-1)
Spray drying has advantages in processing heat-sensitive substances and drugs with a high melting point. Key factors in employing this method are the selection of polymers, solvent types, solvent solubility, and drying conditions. The advantages and disadvantages of spray drying technology are outlined below:
1. Requires a small amount of Active Pharmaceutical Ingredient (API) in the early stages of development, with just gram-level materials sufficing.
2. Enhances the solubility of APIs that are heat sensitive or have high melting points.
3. The method supports the use of diverse solvent systems to optimize dissolution.
4. The resulting spray-dried intermediate boasts excellent compressibility, aiding in subsequent granulation and tablet compression processes.
5. The particle size of the spray-dried intermediate is generally consistent. By adjusting process parameters, it is possible to produce particles of varying sizes and shapes, which provides flexibility in the final drug formulation.
1. Higher Costs: Spray drying requires relatively expensive equipment and consumes a considerable amount of organic solvents. This makes the process both energy-intensive and costly.
2. Scale-Up Equipment Dependency: Maintaining consistent characteristics like form, size, and density during scaling up can be challenging and heavily reliant on the equipment used.
3. Lower Solvent Efficiency: Due to limitations in solubility and viscosity, the solid content in the solution is typically below 10%, resulting in lower efficiency in solvent utilization for spray-dried solids.
4. Environmental Concerns: Spray drying is less environmentally friendly due to the use of organic solvents. It demands specific facility configurations and management practices. Despite closed-loop systems, recycling many organic solvents is challenging. Additionally, numerous low-boiling-point organic solvents are toxic, flammable, and necessitate specialized explosion-proof facilities.
(Note: Ongoing advancements in spray drying technology and processes are gradually mitigating these disadvantages. Given its applicability to a broad range of drug molecules and its intrinsic benefits, spray drying has become the most widely adopted method for production of amorphous solid dispersions.)
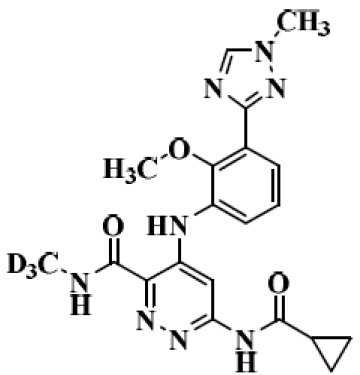
The structure of Deucravacitinib
In September 2022, the US FDA approved Deucravacitinib for treating adults with moderate-to-severe plaque psoriasis, suitable for systemic therapy or phototherapy. This first-of-its-kind, highly selective, oral TYK2 (tyrosine kinase 2) inhibitor, marketed as Sotyktu® with a dosage strength of 6 mg. The Originating company, Bristol Myers Squibb (BMS), forecasts that Sotyktu® will achieve peak sales of $4 billion.
Deucravacitinib, a drug with poor solubility, falls under BCS Class II category, which signifies low solubility and high permeability. Its solubility varies depending on the pH level, it is more soluble at a pH of 1.05 (over 3mg/mL) and less soluble at a pH of 6.5 (only 0.009 mg/mL). To enhance its solubility and minimize the impact of pH variations, BMS explored different salt forms and crystalline polymorphs, ultimately finding that the hydrochloride salt form not only had a weaker pH effect but also offered higher bioavailability compared to the free base and other salt forms. In the dog studies, the hydrochloride salt form demonstrated an average bioavailability that was 60% higher than the free base. Consequently, the hydrochloride salt form was chosen for the phase I and phase II clinical trials, and its use in capsule dosage form.
The hydrochloride salt form of Deucravacitinib tended to undergo racemization, necessitating refrigeration for the capsules over extended periods to prevent conversion to the free base during storage. This significantly raised storage costs and added to the inconvenience. To enable room temperature storage for prolonged durations while retaining good bioavailability and reducing pH sensitivity, a novel formulation strategy was employed in phase III clinical trials – preparing an amorphous solid dispersion (ASD). In this process, the free base crystal form of the drug was mixed with hydroxypropyl methylcellulose acetate succinate (HPMCAS) in a solvent mixture of acetone and water to form a solution. The solution was then spray-dried to create an ASD. Following this, the ASD was mixed with excipients, underwent granulation, milling, blending, and was finally compressed into final products—film-coated immediate-release tablets. This new formulation managed to achieve an absolute bioavailability of up to 99%.
In this case, the innovator initially selected the hydrochloride salt form of Deucravacitinib for its enhanced bioavailability and lesser pH dependence. Subsequent research, however, highlighted the risk of racemization in the hydrochloride salt formulations, raising stability concerns. As a result, the decision was made to use the free base crystalline form. To enhance solubility and bioavailability, an amorphous solid dispersion was developed using spray drying technique. Following these developments, the drug received approval.
The core team of Crystal Formulation Services (a subsidiary of Crystal Pharmatech) boasts experts with backgrounds in polymer materials, chemical engineering, and mechanics. The team’s approach is rooted in rheology and engineering principles, combining deep theoretical knowledge with extensive experience in large-scale processes. This expertise has been instrumental in successfully launching major spray-dried pharmaceuticals in the market. Equipped with top-tier machinery and analytical instruments, ranging from small to production scale, the team is well-positioned to support global pharmaceutical companies delivering high-quality drugs developed through spray drying.
1. A Review of Various Manufacturing Approaches for Developing Amorphous Solid Dispersions
2. WO2019232138A1
3. WO2021055651A1
4. EMA Public Assessment Report
Subscribe to be the first to get the updates!