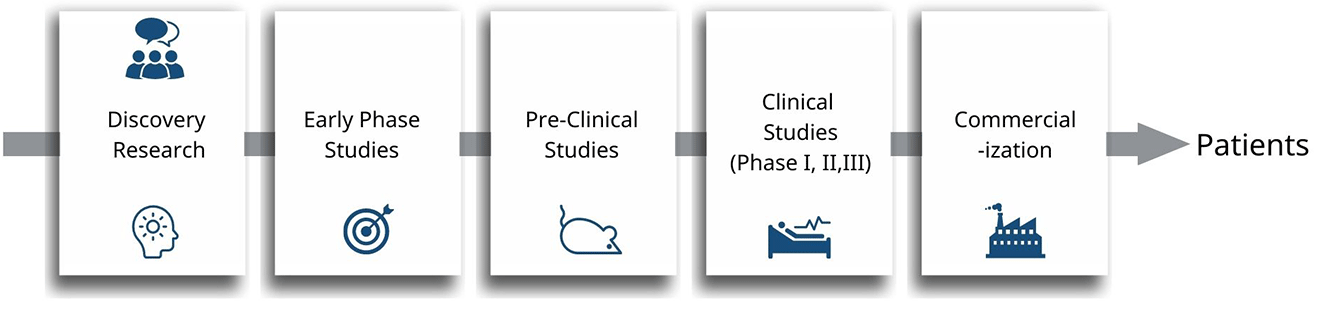
Characterization of biologics plays an important role in the development of Biopharmaceuticals, such as monoclonal antibodies, fusion proteins, antibody drug conjugates, biosimilars etc. Characterization is the analysis of biologics to understand the primary, secondary, tertiary, and other higher order structure using physicochemical, biophysical and biological methods. During biologics drug development process, physicochemical, biophysical and biological properties can have an impact on the product's performance and safety.