Hot melt extrusion (HME) technology is widely used in the development of amorphous solid dispersions (ASD) due to its advantages such as solvent-free processing, continuous manufacturing, high automation, excellent process reproducibility, and the ability for large-scale production. Polymers play a crucial role in the formulation of polymer-drug amorphous solid dispersions, contributing to structural stability, enhanced solubility, compatibility control, and prevention of phase separation. The choice of the appropriate polymer is essential for achieving physical stability and desirable in vitro performance of amorphous drugs. This article will provide insights into selecting suitable polymers when preparing amorphous solid dispersions through hot melt extrusion.
Selecting the right polymer for hot melt extrusion (HME) is crucial to ensure the success of the formulation and processing. HME involves the melting and extrusion of drug-polymer blends, and the choice of polymer should meet specific criteria:
Thermoplastic Properties: The selected polymer must exhibit thermoplastic behavior, meaning it can soften and deform when heated. This property is essential for processing through the HME equipment.
Glass Transition Temperature (Tg): The polymer's Tg should be within the appropriate range for the extrusion process. Typically, polymers with Tg values between 50°C and 180°C are suitable. Tg should be compatible with the processing temperature but still allow for efficient extrusion.
Thermal Stability: The chosen polymer should remain stable and not undergo decomposition or degradation at the temperatures used during extrusion. Ensuring the stability of the polymer throughout the process is essential to prevent the formation of toxic decomposition products.
Melt Viscosity: Polymers with moderate to low melt viscosity are preferred for HME. Most polymers exhibit pseudoplastic behavior, meaning their viscosity decreases as shear stress increases. This property improves the flowability and processability of the polymer in the extrusion equipment.
Hygroscopicity: Low hygroscopicity is desirable in a polymer to minimize the impact of moisture on the stability and performance of the final amorphous solid dispersion. While high temperatures during HME can help remove absorbed moisture, excessively hygroscopic polymers may still pose challenges.
Solubility: The polymer's ability to dissolve or be compatible with the drug substance is critical. A polymer with good solubility for the drug will enhance drug dispersion and dissolution within the polymer matrix.
Regulatory Requirements: Ensure that the selected polymer complies with relevant regulatory standards, especially if the polymer will be used in a pharmaceutical product. Use non-toxic polymers that meet human-use standards and have obtained the necessary approvals.
Plasticizers: Consider whether the addition of plasticizers may be necessary to modify the polymer's Tg or other properties to better suit the HME process.
The choice of polymer should align with the specific characteristics of the drug substance, the processing conditions, and the desired properties of the amorphous solid dispersion. Compatibility between the polymer and the drug is crucial to prevent phase separation or drug crystallization. Extensive compatibility studies, including thermal analysis and miscibility tests, should be conducted during the formulation development process to ensure the chosen polymer meets the required criteria for the successful preparation of amorphous solid dispersions through HME.
In some cases, using a combination of polymers can enhance their properties to meet specific formulation requirements. Here are several commonly used polymers for HME:
Polyethylene-Related Polymers
Polyvinylpyrrolidone (PVP), also known as Povidone, is a high-molecular-weight polymer formed by the polymerization of N-vinyl-2-pyrrolidone. PVP contains amide bonds that can participate in hydrogen bonding interactions with drugs, enhancing wetting and inhibiting drug crystallization. However, high-molecular-weight PVP typically has a high glass transition temperature (Tg), making it unsuitable for hot melt extrusion (HME) of solid dispersions.
PVP/VA (Polyvinylpyrrolidone/Vinyl Acetate Copolymer), also known as Copovidone, is a copolymer of vinylpyrrolidone and vinyl acetate. By introducing flexible vinyl acetate monomers, Copovidone significantly lowers the Tg of the polymer, making it more suitable for HME. It has a broad processing temperature range and excellent rheological properties, making it a preferred polymer for many applications compared to PVP.
Soluplus® is designed specifically for HME and solid dispersion technologies. It is a copolymer created by grafting hydrophobic vinyl caprolactam monomers and vinyl acetate monomers onto a hydrophilic PEG backbone. Soluplus® can form micelles in water, enhancing solubilization efficiency. The presence of vinyl acetate and PEG reduces the Tg of the copolymer, allowing for extrusion at lower temperatures, which is advantageous for processing heat-sensitive drugs.
Cellulose-Derived Polymers
Hydroxypropyl Methylcellulose (HPMC) is a non-ionic cellulose ether and a semi-synthetic, non-reactive, viscoelastic polymer. It is used to improve drug stability and control drug release in various formulations. HPMC is suitable for both immediate-release and sustained-release formulations.
Hydroxypropyl Methylcellulose Acetate Succinate (HPMCAS) is a copolymer of hydroxypropyl methylcellulose with acetate and succinate groups. HPMCAS exhibits amphiphilic properties. Its degree of acetylation and succinylation affects drug release behavior and crystallization inhibition at different pH values. L-type HPMCAS, with the highest succinyl content, is hydrophilic and promotes rapid drug release in the gastrointestinal tract.
Other Polymers
Polyethylene Glycol (PEG) is a versatile polymer used for preparing solid dispersions of poorly water-soluble drugs. It offers good water solubility, safety, low melting points for medium to high molecular weight PEG, and stability. PEG can be used alone or as a plasticizer mixed with other polymers to improve drug solubility and wetting properties.
Eudragit® is a common family of methacrylic acid copolymers used in various pharmaceutical applications. Depending on the functional groups, Eudragit® polymers can provide gastric resistance, enteric properties, or permeability control for drug release. Eudragit® EPO, which promotes drug release and is suitable for HME, is a pH-sensitive water-soluble polymer.It is soluble in solutions with a pH below 5 and swells in solutions with a pH above 5. It has a glass transition temperature (Tg) of approximately 50°C and exhibits good thermoplasticity, making it suitable for the preparation of amorphous solid dispersions.
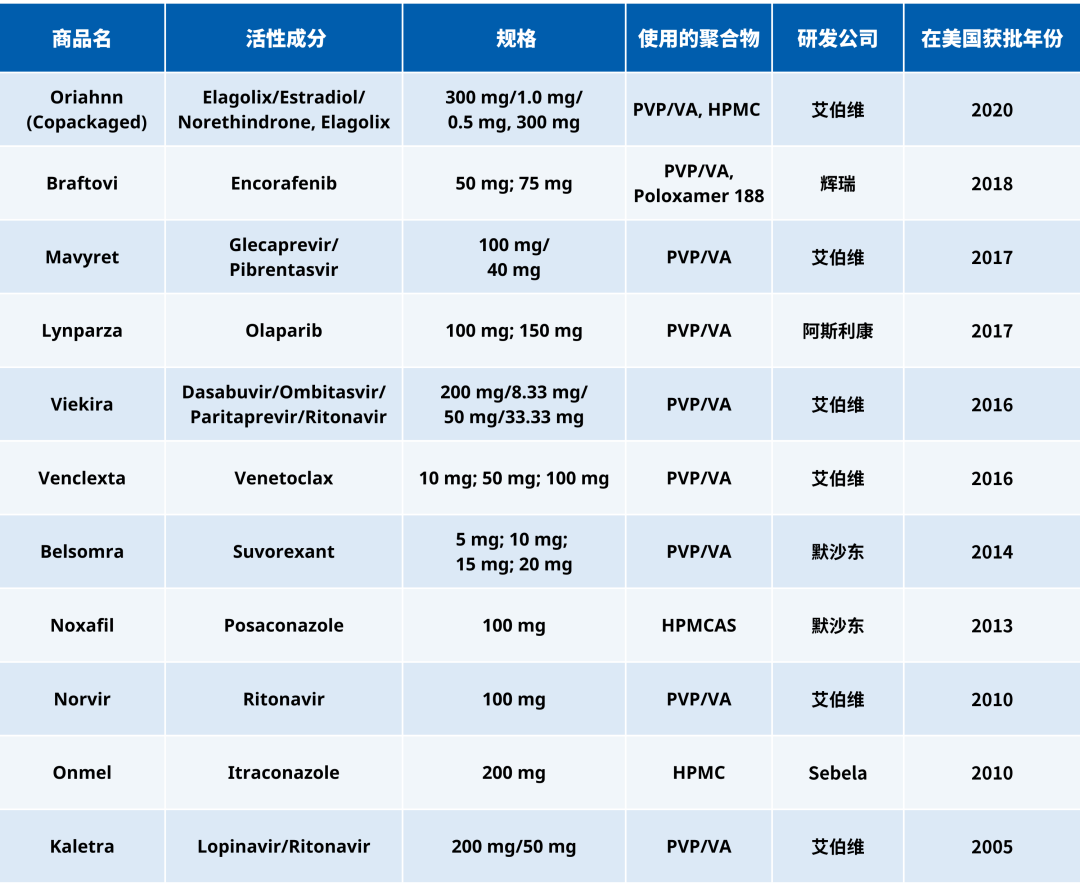
Table 1 lists some of the pharmaceutical drugs approved by the U.S. FDA in the past 20 years that were prepared using hot melt extrusion (HME) technology to create amorphous solid dispersion formulations. Among these, PVP/VA is one of the most commonly used polymers.
HPMCAS's application cases in hot melt extrusion
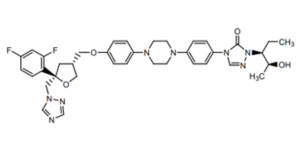
The chemical structure of Posaconazole
Posaconazole is a second-generation triazole antifungal medication used for the prevention and treatment of invasive fungal infections. It is available in various formulations, including oral suspension, delayed-release tablets, and intravenous injection. Posaconazole has a weakly alkaline pH and low solubility. Its solubility decreases rapidly as the pH of the medium increases, and in media with a pH greater than 5, its solubility can drop to less than 1 μg/mL. This low solubility limits its absorption in the gastrointestinal tract.
To enhance the absorption of Posaconazole, patients taking the oral suspension are often advised to consume a high-fat meal or nutritional supplement simultaneously to prolong its gastric residence time.
In contrast, Posaconazole delayed-release tablets use a pH-dependent hydroxypropyl methylcellulose acetate succinate (HPMCAS) as a carrier to improve its bioavailability. This technology, based on hot melt extrusion (HME) with pH-dependent polymers, allows for increased drug absorption without the need for high-fat meals. HPMCAS, especially the M-type, effectively inhibits the recrystallization of Posaconazole and maintains it in a saturated state for an extended period. In vivo studies have shown that Posaconazole delayed-release tablets significantly enhance bioavailability and reduce the impact of food on blood drug concentrations compared to the oral suspension.
HPMCAS provides enteric solubility, preventing Posaconazole from dissolving in the stomach. This not only reduces the influence of gastric contents on Posaconazole but also prevents recrystallization. Additionally, succinyl groups in HPMCAS can form ionic interactions with Posaconazole, further promoting its dissolution and absorption in the intestines, leading to a significant increase in blood drug concentration and bioavailability.
The choice of suitable polymers remains a crucial factor in the successful development of amorphous solid dispersion formulations. While there are currently limited polymer options for commercial production using hot melt extrusion, ongoing polymer research may expand the applications of HME technology in the development of solid dispersion formulations.
Subscribe to be the first to get the updates!