Oxybutynin HCl (Fig. 14) has been recognized as a BDDCS I compound exhibiting high solubility and permeability (Benet et al. 2011). It has been used in a variety of marketed products for the treatment of overactive bladder (Gamble and Sand 2008). The first oral formulation from Hoechst Marion Roussel in 1975 was an immediate release tablet (Ditropan®), which was dosed three times a day. The major side effect was dry mouth, which was the primary reason for patients discontinuing use (Sathyan et al. 2001). The side effect of dry mouth was caused by the metabolite desethyloxybutynin. The metabolite was reduced by developing a controlled release dosage form, which maintained a zero order release. This resulted in lower peak to trough variations in plasma levels and bypassed the pre-systemic metabolism and conversion to the active metabolite. Ditropan XL® was launched in 1999 using Alza’s osmotic delivery (OROS) formulation approach, which reduced the severity of dry mouth side effects (Sathyan et al. 2001). This formulation approach also allowed one daily dose, as opposed to the original three daily doses, which was more convenient for the patient and helped improve patient compliance.
Fig. 14: Structure of oxybutynin HCl
Another way to reduce the metabolite and side effects was to bypass the first pass metabolism using a different administration route. Watson launched an Oxytrol® transdermal patch in 2003, which was designed to deliver oxybutynin over a three to four day interval. Reformulation into the patch required researchers to use the oxybutynin free base, instead of the hydrochloride salt, for better skin transport. Bypassing the oral delivery route significantly reduced the metabolite (Fig. 15), which resulted in minimal side effects and better patient compliance (Gamble and Sand 2008). In January 2013, an over-the-counter (OTC) patch was approved by the FDA for commercial use (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm336815.htm. Accessed 2 March 2015).
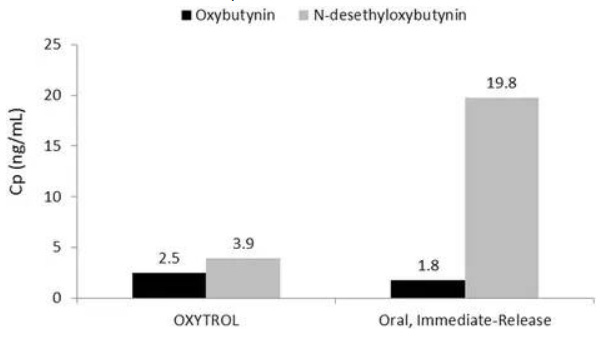
Fig. 15: Comparison of blood levels of oxybutynin and its metabolite, N-desethyloxybutynin, showing that the patch resulted in significantly lower levels of the metabolite, which reduced the dry mouth side effect, compared to the oral immediate release formulation (Oxytrol Package Insert. (available at http://pi.watson.com/data_stream.asp?product_group=1295&p=pi&language=E. Accessed 2 March 2015)
This case study shows how a change in form and delivery route has not only reduced side effects, but also resulted in a more efficient and convenient drug product for the patient. The development of the patch required a change in form from the hydrochloride salt to free base, which enabled the drug to pass through the skin. Finding a different form to develop an improved drug product required an understanding of the properties needed for a particular dosage form and thorough characterization of various forms. This change in form could include a polymorph, free acid/base, salt, cocrystal, or amorphous solid dispersion. Specific counterions or guest molecules would need to be considered for certain delivery routes, such as dermal, ophthalmic, intravenous, or intramuscular formulations (Paulekuhn et al. 2007). Determining the issues with current products and finding creative solutions using form and formulation to produce an improved product has been recognized as a true “win-win” in lifecycle management.
Conclusions
The case studies in this manuscript have been presented to show why it has been critical to characterize, understand, and monitor the solid form in all stages of drug discovery and development. While these case studies have been presented in the literature, there have been even “uglier” cases that have not been published. It is important for researchers to realize that form selection is not a unit operation, but an integral part of the entire drug development process, with no clear beginning or end; instead, there should be continuous scrutiny and monitoring as a candidate progresses from discovery to development to market and beyond.
Subscribe to be the first to get the updates!