Gatifloxacin (also known as AM-1155, CG5501, and BMS-206584) has been established as a fluoroquinoline broad-spectrum antibiotic (Fukuda et al. 1998) (Fig. 10). It was originally discovered by Kyorin Pharmaceuticals in the late 1980s as a hemihydrate that was recrystallized from methanol (Masuzawa et al.1991). This crystalline form was found to be hygroscopic and resulted in poor tablet disintegration and dissolution. In the mid-1990s, a sesquihydrate was found by Kyorin with improved properties (Matsumoto et al. 1999). The compound was licensed to Bristol-Myers Squibb (BMS) in 1996 with two hydrated forms disclosed.
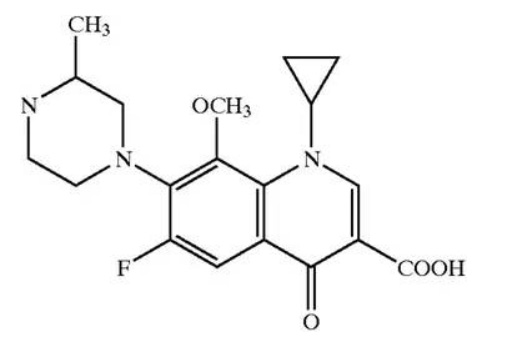
Fig. 10: Structure of gatifloxacin
Initial clinical formulations at BMS utilized the sesquihydrate in a wet granulation process. The clinical batch failed specifications when a new crystal form was discovered in the batch. The new crystal form was confirmed as a pentahydrate (Raghaven et al. 2002), which was found to be less soluble and more stable in various formulations (wet granulations, dry blends, and aqueous suspensions). Issues with the initial clinical sesquihydrate formulation, as well as difficulty producing pure sesquihydrate material, had prompted crystallization studies to find a better understanding of the solid form landscape.
Crystallization studies using only ethanol, water, and various drying conditions resulted in 12 additional forms for gatifloxacin (Fig. 11) (Raghaven et al. 2002). These studies added considerable elements to the development timeline of the compound, including finding the forms, developing API processes for the desired forms, optimizing clinical formulations, and requalifying analytical methods. While the pentahydrate exhibited superb physical properties for the API and formulation, it was also found to be less bioavailable compared to the sesquihydrate. This resulted in a switch back to the sesquihydrate form for the marketed tablet formulation Tequin®, approved in 1999 (Fish and North 2001). Potentially fatal blood sugar problems resulted in a blackbox warning for Tequin®, as well as a subsequent removal of Tequin® from the US and Canadian markets in 2006. The sesquihydrate was subsequently used in the production of ophthalmic solutions, Zymar® and Zymaxid®. After the compound patent had expired in 2010, Apotex started to use the hemihydrate in their generic product.
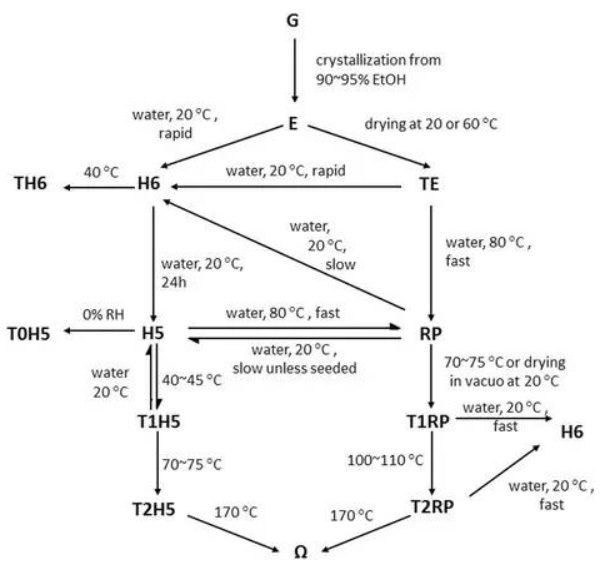
Fig. 11: Gatifloxacin crystalline forms from ethanaol, water, and various drying conditions (adapted from reference (Raghaven et al. 2002))
Gatifloxacin has provided an example of multiple form changes throughout mid to late stage development. These changes created significant additional work around API crystallization development, formulation processing, analytical methods, and biological studies (i.e., bridging and bioequivalence studies). This case study has also demonstrated the criticality of due diligence for in-licensed compounds, including proper screening. Companies that in-license a compound should ask specific questions about the solid form studies that were performed to determine the scope of knowledge and inter-relationships between forms, and how the solid form landscape would impact the desired dosage form and development plan. For companies that out-license a compound, a solid form study targeted toward the most stable form, crystallization conditions, and formulation processes have resulted in a much stronger package.
While the initial package for gatifloxacin seemed straightforward with only two hydrated forms, it should be classified as an "ugly" scenario due to the solid form changes and additional studies.
Subscribe to be the first to get the updates!