Background
It has been reported that the solid form of active pharmaceutical ingredients (APIs) has significantly impacted quality and consistency of the final dosage form for drug development compounds (Newman and Byrn2003), especially for solid oral dosage formulations. Therefore, monitoring and controlling the API solid form in both drug substance and drug product has been recommended in order to ensure consistent biopharmaceutical properties throughout a drug development program.
Every innovator drug developer has approached API solid form decisions with a unique paradigm; however, identifying and maintaining the optimal API solid form in early pharmacokinetic studies, as well as maintaining this form through product launch, has been recognized as an ideal situation. This utopian scenario, however, has often been noted to be far removed from reality, especially if the API solid form has been ignored or assumed to be trivial for a particular program. This has often led to significant program delays and cost as bioequivalence studies, new crystallization studies, or formulation development may have been needed.
This manuscript presents the “good, bad, and ugly” aspects of API solid form changes in the pharmaceutical industry. It has explored and elaborated upon specific case studies that outline the impact of API solid form changes brought about by choosing a non-ideal salt form for early preclinical development, relaxed due-diligence for a “fast-tracked” compound, a serendipitous late stage form change, lack of attention to solid form for an in-licensed compound, and a less than bullet-proof intellectual property (IP) landscape surrounding an innovator molecule.
The goal of these examples was to show that adequate attention to API solid form during development will aid in managing risk for a program. Whether an innovator company was looking to out-license a gold molecule as a platinum package, an innovator company was looking to bring a drug to market with a strong patent landscape, or a generic company was looking to enter the market with IP for their molecule, the case studies presented in this manuscript clearly show that API solid form is an important aspect of any development program.
The case studies presented, in addition to many other un-published examples, have confirmed to pharmaceutical scientists that no screening strategy can guarantee that all crystal forms have been discovered. However, appropriate attention to API form and a sound screening strategy has had the potential to mitigate the risk for form changes in the API and drug product.
Solid forms have been defined as both crystalline and amorphous materials in this paper. Crystalline forms have been sub-classified into categories outlined in Fig. 1, and described as neutral (such as free forms and co-crystals) and charged (salts or salts of co-crystals) species. Each category of crystalline materials has the possibility of displaying polymorphism (solvates and hydrates have been included in our polymorph classification based on the regulatory definition). Any material from the crystalline API categories that lacks long range order as characterized by x-ray powder diffraction (XRPD) has been referred to as amorphous API.
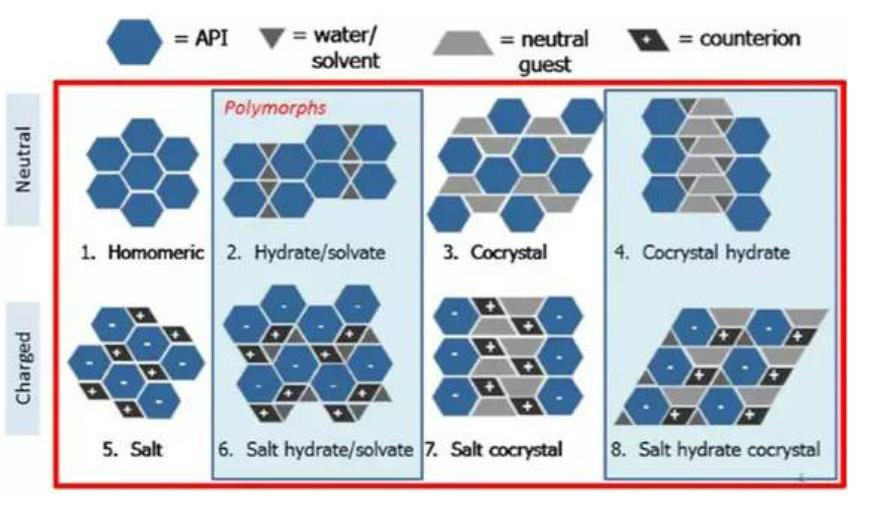
Fig. 1 Classification of crystalline API forms. The red box signifies that polymorphs of all solid form classes are possible; the blue boxes represent classes containing hydrates
Case studies 1
Indinavir - early salt form change
Indinavir sulfate, marketed as Crixivan® (Fig. 2), was approved in 1996 as a human immunodeficiency virus type 1 (HIV-1) protease inhibitor indicated for treatment of HIV infection and AIDS in adults (Lin 1999; Lin et al. 1998; Crixivan Package Insert. (available at http://www.merck.com/product/usa/pi_circulars/c/crixivan/crixivan_pi.pdf. Accessed 23 Feb 2016). Crixivan® was initially developed as a free base monohydrate, but suffered from significant pH dependent solubility (Fig. 3) and limited adsorption as the free base form (Lin et al. 1998). As a result, a need to identify an acceptable, soluble salt for clinical dosage development arose for researchers. The pH solubility profile and pKa of the molecule suggested a rather acidic salt was necessary to achieve complete dissolution. One issue, however, was that Crixivan® was quite unstable in acidic solutions (Table 1), which presented a stability risk for solid salt forms (Lin et al. 1998). The crystalline sulfate salt ethanolate was chosen as the lead salt form for development. The aqueous solubility for this salt form was in excess of 500 mg/ml with a resulting solution pH of < 3. The main concern for the sulfate salt ethanolate was the excessive hygroscopicity (Fig. 4). Additionally, the ethanolate had the potential to change physical form at elevated humidity, even potentially going amorphous. Because of this, extensive solid-state stability and excipient compatibility studies were performed using controlled humidity conditions. Experiments showed that a shelf life of > 2 years was possible when the humidity was kept < 30 % relative humidity (RH), even for the amorphous sulfate salt. At temperatures and humidity above 40 °C and 30 % RH respectively, the sulfate salt suffered from rapid degradation for both the API and drug product. Because of the need for low RH, a dry granulation formulation process was developed for the drug product (Lui et al. 2003). Human clinical trials were conducted with both the sulfate salt ethanolate and free base monohydrate (Yeh et al. 1998). The study showed that the sulfate salt in the fasted state or with a low fat meal yielded the highest exposures (Fig. 5).
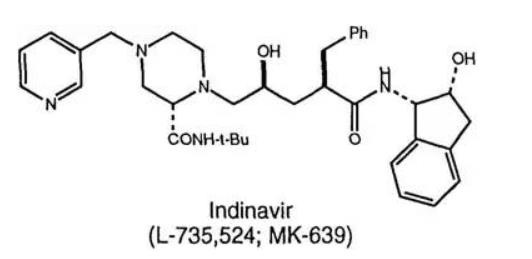
Fig. 2 Structure of indinavir
Fig. 3pH solubility profile of indinavir (adapted from Ref (Lin et al. 1998))
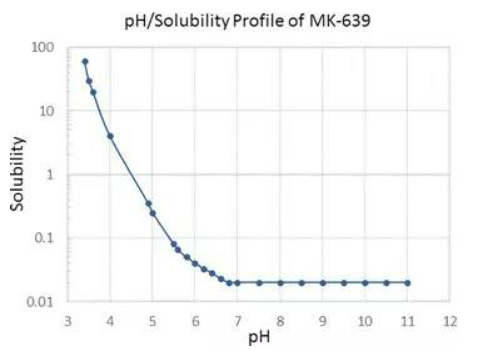
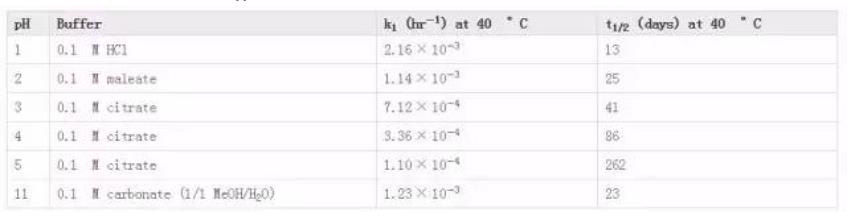
Table 1pH Stability Data for Indinavir (used with permission from Ref (Crixivan Package Insert. (available at http://www.merck.com/product/usa/pi_circulars/c/crixivan/crixivan_pi.pdf. Accessed 2 March 2015))
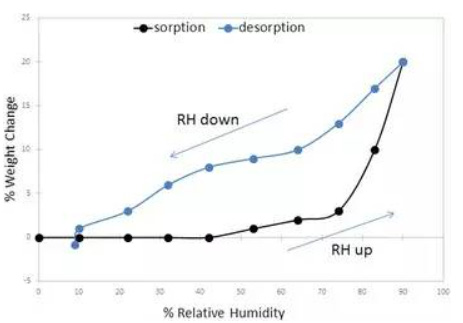
Fig. 4Water sorption/desorption profile of indinavir (adapted from Ref (Lin et al. 1998))
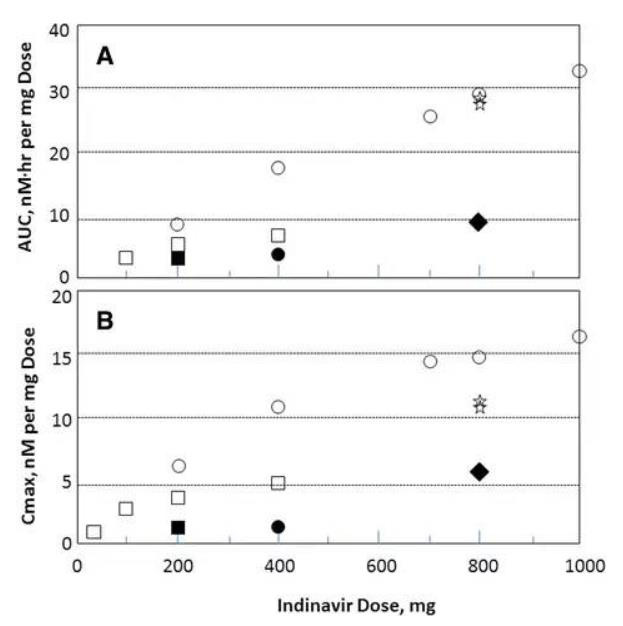
Fig. 5AUC (a) and Cmax (b) curves for indinavir as a function of the administered single-dose; AUC and Cmax values were normalized to a 1-mg dose. ○ sulfate salt fasted state; ● sulfate salt following a high-fat meal; ☆sulfate salt following low fat meal; □ free-base capsules fasted state; ▪ free base capsules following a high-fat meal; ♦ free base oral suspension fasted state (used with permission from Ref (Yeh et al. 1998))
This example has clearly displayed the utility of identifying the appropriate salt form before clinical trials have been initiated and has also represented a “good” scenario for solid form in development. This case study has presented a classic example of solid state form impacting pharmacokinetic profiles of a drug. The example has also shown that relatively poor physicochemical properties can be mitigated with a thorough understanding of both chemical and physical stability profiles. The sulfate salt ethanolate displayed excessive hygroscopicity and form change potential; however, processing and storage conditions were identified to successfully process and store API and drug product.
Subscribe to be the first to get the updates!